“Uniqueness of H2O”
advertisement

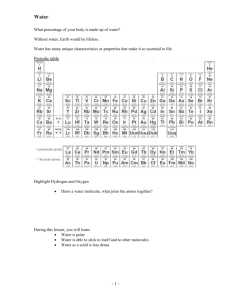
“Uniqueness of H2O” Activity 1: Materials needed: balloon, cloth and water faucet (sink) 1. Turn on the water so a small stream is flowing. 2. Rub the balloon on hair or a cloth several times to build up a static charge on the balloon. 3. Place the charged balloon close to the stream of water, but do not let the balloon touch the water. 4. Observe the effect of the charged balloon on the stream of water. Activity 2: Materials needed: 10 test tubes, water, oil, alcohol, lighter fluid, , and salt 1. Put equal amounts of oil into 5 test tubes and equal amounts of water into 5 test tubes. 2. Test the solubility of each of the solvents (water & oil) by adding a different solute to a water tube and an oil tube. 3. Gently swirl each tube to mix the contents. 4. Judge how well each solute dissolved in the water and oil and record on your data sheet. Be prepared to share your data with the other class members. Activity 3: Materials needed: penny, beaker of H2O, pipette, paper towel 1. Place the penny on the paper towel, on a flat surface. 2. Predict how many drops of H2O the penny will hold without overflowing. Record this on your data sheet. 3. Add H2O drop by drop to the surface of the penny. Observe the shape the water takes on top of the penny. Record your observation. 4. Count the number of drops added before the water overflowed. 5. Record this number on your data sheet. 6. How many paper clips do you think will float on a surface of water? Record on data sheet. Now, using a fork, gently place a paper clip on to the surface of the water in the large beaker. Count the number of paper clips and record on your answer sheet. Activity 4: A. 1. 2. 3. Materials needed: glass slide, capillary tubing, pipette, and beaker of H 2O Put a drop of colored water on the glass slide. Place one end of the capillary tubing in the H2O. Record your observations on your data sheet. B: Demo 1. At the front of the room, observe the graduated cylinder containing the H 2O. The curved surface of the H2O is called the meniscus. Draw the shape of the water meniscus on your data sheet. Activity 5: Materials needed: dropper bottle of H2O, dropper bottle of alcohol, paper towel 1. Place a drop of H2O on the paper towel. Circle it with a pencil, and label it as H 2O. 2. Place a drop of alcohol on the paper towel. Circle it with a pencil, and label it as alcohol. 3. Each minute for 5 minutes observe the drops and record your observations on your data sheet. Activity 6: Materials needed: beaker with H2O and ice, frozen bottle of water, beaker with ice and water 1. Observe the bottle of frozen and the bottle of liquid H2O. Record your observations on your data sheet. 2. Observe the position of the ice in the beaker. Record your observation on the data sheet. “Uniqueness of Water” Activity 1: 1. What happened to the stream of water when the charged balloon was close to it? 2. What does this tell you about the water molecules? Activity 2: Record the solubility of the solvents in water and oil. S = soluble I = insoluble Water Oil Polar or Nonpolar Alcohol Lighter Fluid Copper (II) Sulfate Salt 3. The general rule of solubility is “like dissolves like”. In other words, polar solvents dissolve polar solutes and nonpolar solvents dissolve non-polar solutes. If water is polar, do you think oil is polar also? Why or why not? 4. Which of the solutes are polar and which are nonpolar? Record your answer in the appropriate column. Activity 3: 5. 6. 7. How many drops of water do you PREDICT the penny will hold without overflowing? ________ How many drops of water did the penny ACTUALLY hold before overflowing? ________ Draw a picture of the shape of the water on top of the penny before it overflowed. 8. 9. How might the polarity of the water molecule explain the behavior you just observed? Predicted number of paper clips ________ Actual number of paper clips _________ How is this possible if the density of the paper clip is greater than that of water? Activity 4: 10. Draw the shape of the water meniscus in the graduated cylinder. 11. Xylem tubes are tiny tubes in plants that run from the roots to the leaves. Their job is to transport water. How is it that water flows against the gradient of gravity from the roots to the leaves? Observe the stalk of celery at the front of the room. 12. Adhesion and cohesion are forces responsible for the meniscus and capillary action. Explain the difference in the two forces and how they are responsible for capillary action. Activity 5: Rate the moisture level of the water and alcohol at one-minute intervals for 5 minutes. ++for very moist +moist - dry - - for very dry 1 min 2 min 3 min 4 min 5 min Water Alcohol 13. How does this property result in the cooling effect when humans sweat or dog’s pant? Activity 6: 14. How does the volume of freezing water compare to the volume of liquid water? How do you know? 15. What does this tell you about the density of liquid water compared to frozen water? (D = m/v) 16. How is this characteristic an advantage for aquatic organisms in a very cold environment such as the tundra?