Properties of Water Lab
advertisement

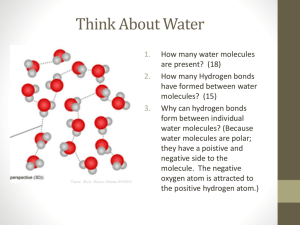
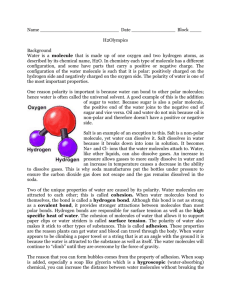
Water What percentage of your body is made up of water? Without water, Earth would be lifeless. Water has many unique characteristics or properties that make it so essential to life. Periodic table Highlight Hydrogen and Oxygen Draw a water molecule, what joins the atoms together? During this lesson, you will learn: Water is polar Water is able to stick to itself and to other molecules Water as a solid is less dense -1- Water Water is Polar What happens when you rub a balloon? What happens when you hold that balloon up against a stream of water? Water is a polar molecule, what does this mean? Add the appropriate notation to a water molecule above. Polar Compounds are unique in that they often have properties that differ from the properties of the elements of which they are composed. This is definitely true of water. Element – Compound – Mixture - Describe 2 differences between the compound water and the elements of which it is composed. Water is formed of oxygen and hydrogen atoms in the ratio, 2 hydrogens to 1 oxygen. You can write the chemical reaction which creates this. However all chemical reactions must be balanced, what goes in must come out! Which is an example of a balanced equation? o o o o 2 H2 + O2 → 2 H2O H2 + O2 → 2 H2O 2 H2 + O2 → H2O 2 H2 + 2 O2 → 2 H2O Water sticks to itself and other molecules There are 3 tubes filled with water what do you notice about them? If you look closely, you will notice that the top of the water where it touches the tube is creeping up the sides, rising above the rest of the water. In chemistry, we call this effect -2- Water the meniscus. This effect is due to the presence of charged molecules in the wall of the straw. How would charged molecules in the tubes allow the water to creep up the sides of the straw? What impact does the diameter of the tube have on the phenomenon you observe? Why? Two straws removed from water with finger over the top. What happens with the large straw? How about the small straw? Explain why the water in the small straw behaves differently than the water in the large straw. This is all because of two processes: cohesion – the ability of water to stick to itself and adhesion – the ability of water to stick to other molecules. Cohesion and adhesion play a very important role in the natural world. In fact, plants would not be able to survive (much less us) without these properties of water. Can you think about how this might be the case? Surface Tension Observe the Petri dish on the table. What is unique about this situation? The same forces that allow the needle to float on the water are responsible for the niche of the water strider and other small surface-dwelling insects. Using the pipette on the table, I will drop water onto the surface of the penny. The challenge is to fill the top of the penny with as many drops as possible. -3- Water Who wants to try? Were you surprised by the number of drops of water that we were able to put onto the penny? Why or why not? Explain what occurs at a molecular level to allow so much water to stay on top of the penny. Try the penny procedure a second time using alcohol instead of water. What do you observe? Why do you think this is the case? Why is it important that the water reaches a point where it spills off the penny? In your answer, provide a few examples of the problems that would be associated with water that is too “sticky”. Stickiness = Hydrogen Bonds – draw 3 water molecules joined by hydrogen bonds -4- Water Ice What happens as we cool a substance? How will this affect the hydrogen bonds? Draw a picture to describe what is going on: What happens when you make ice lollies? Why is this property of water of advantage to living things? -5-