Preparing A Bacterial Smear
advertisement

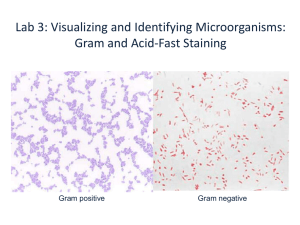
1 Preparing a Bacterial Smear -Since bacterial cells are so small, special problems arise in the preparation of a good smear -There are 3 principal precautions that you must take to avoid problems: 1. Do not use scratched microscope slides since scratches in the glass slide can be confused with microorganisms. 2. Be sure that you are working with a very clean slide. 3. Avoid making smears that are too thick or too thin. A dirty slide may be greasy or may be laden with dirt and dust; such a slide will result in an unsatisfactory smear for 3 reasons: 1. The smear containing the desired microbes will wash off the slide during the staining process. 2. When you deposit the bacterial suspension on the microscope slide, it will coalesce, i.e., it will not remain spread out. 3. Dirt, dust and other debris can be mistaken for microbes. -the slide must be so clean that a drop of water spreads out on it *water coalescing/beading on the slide indicates the presence of an oily film that clumps bacteria *even a small amount of oil from a fingerprint leaves a film *Another difficulty in making a good smear is to get the right amount of bacteria on the slide. a good smear should neither be too thick nor too thin 1. What are the consequences if a smear is too thick? (too many cells on the slide) -does not allow the proper penetration of light through the smear -bacterial cells are often packed too closely together *You are most likely to make thick smears when your cells are obtained with a loop from solid culture media (e.g. slant and agar plate) 2. What are the consequences if a smear is too thin? (only a few cells are deposited on the slide) -searching for bacterial cells is time-consuming *thin smears usually result when the smears are made from broth cultures 2 -LABELING is as important in making smears for stained slides as it is for cultures labeling should be done at one end of the slide -AIR DRYING of the slides should be done in a flat position -HEAT FIXATION kills the microorganisms, coagulates the protoplasm of the cells and causes them to adhere to the slide adequate heat fixation shrinks cells slightly, but it helps bacteria adhere to the slide through several rinses excessive heat warps cells; applying heat to the smear before it is completely dry also distorts cells -Remember to PRACTICE ASEPTIC TECHNIQUE -Smears from Broth Cultures: disperse the bacteria that have settled to the bottom of the broth culture tube -Smears from Growth on Solid Media: *bacteria from the solid agar must be put into a liquid before they can be spread out on the slide add a small amount of NSS/water (2-3 loopfuls or 1 small drop) to the center of the slide -a large drop is undesirable because it takes much longer for the slide to dry aseptically remove only a small amount of bacteria (colony) from the culture mix and spread out Staining Bacterial Smears -The Chemistry of Staining: based on the principle that unlike charges attract; similar charges repel -a bacterial cells carries electrical charges: in an aqueous environment, with the pH at approx. 7, the net electrical charge produced by most bacteria is negative the (-) charged cells attract molecules carrying (+) charges; repel those that are (-) charged 3 -each dye in a salt containing 2 ions—one with a (+) charge (cation), and the other with a (-) charge (anion) either one of the two ions can be the chromophore (part of the molecule that is brightly colored) -the most frequently employed microbiological dyes are called basic dyes— their chromophores are (+) charged the chromophores are attracted to, and subsequently color bacteria examples of basic dyes: methylene blue, crystal violet, safranin acid dyes (e.g. nigrosine) are generally repelled by bacteria because of the (-) charges on their chromophores (acidic dyes color the background and leave the cells colorless) Gram Staining Table 1.1 The Gram Stain Procedure Step Primary Stain Reagent Crystal Violet Time 1 min Color Gram (+) Gram (-) Purple Purple Gram’s Iodine 1 min Purple Purple 95% Ethanol Brief Purple Colorless Safranin 30 sec- Purple 1 min Pink/Red Brief H2O rinse Mordant Brief H2O rinse Decolorizer Brief H2O rinse Counter Stain Brief H2O rinse Table 1.2 How to Limit Variables that Affect Gram Stain Results 1. Use actively growing cells. Old cells lose their ability to hold the stain; they appear gram-negative. 2. Prepare thin smears and adjust decolorization time. Where cells are 3. 4. 5. 6. crowded, they resist decolorization. Bacteria in thin smears decolorize much faster than bacteria in thick smears. Examine well-dispersed cells; avoid stain crystals. The Gram reactions of clumped bacteria or cells adjacent to crystals of dye are unreliable. Avoid overheating the cells during heat fixation. Excessive heat disrupts cell walls making gram-positive bacteria appear gram-negative. Use fresh staining reagents. Old reagents give variable results and form crystals. Adjust timing to your reagents. For example, many laboratories decolorize with fast-acting acetone-alcohol instead of 95% ethanol. Do not rinse too long. Water is a decolorizer. 7. 8. Remember, gram-positivity is a characteristic that can be lost. 4 Acid Fast Staining -in 1882 Paul Ehrlich developed the Acid-Fast Stain while working with Mycobacterium tuberculosis -the Acid-fast procedure is a differential stain one that distinguishes one group of bacteria from another -Mycobacterium and many Nocardia species are called acid-fast because during the staining procedure, they retain the primary dye despite decolorization with the powerful solvent acid-alcohol nearly all other genera are non acid-fast -Ehrlich’s method was improved by later microbiologists including Ziehl and Neelsen both the Ehrlich and the Ziehl-Neelsen techniques require heat to drive a primary dye past the cell wall Kinyoun acid-fast Procedure, a wetting agent (detergent) makes heating unnecessary -Cell wall structure and Acid-Fast Staining: in the cell walls of Mycobacterium and many Nocardia species are lipoidal mycolic acids *normally, these lipids prevent dye chromophores from coloring the cell *some mycobacteria, including Mycobacterium tuberculosis contain so much mycolic acid that they are nearly impossible to stain with Gram Staining *others e.g. M. smegmatis (originally isolated from smegma) and M. phlei (isolated from hay and grass) are protected by less mycolic acid one theory concerning the ability of the acid-fast stain to differentiate bacteria holds that mycolic acids are somewhat permeable to dye that is dissolved in alcohol and phenol and applied with heat (or detergent) -after the bacteria are cooled (or the detergent is rinsed off), mycolic acids in cell walls coalesce, forming a barrierthe lipids prevent acid-alcohol from decolorizing the protoplasm -Medical Significance of Acid-fast Bacteria and Acid-fast Staining 5 Table 1.3 The Ziehl-Neelsen Acid-Fast Stain Procedure Procedure Reagent Time Cell Color Acid-Fast Primary Dye Carbolfuchsin 3-5 Brief H2O rinse (contains basic fuchsin, phenol, ethanol and H2O) mins. Decolorizer Brief Acid-alcohol Non Acid-Fast Red Red Red Colorless Brief H2O rinse (3% conc. HCl in 95% ethanol) Counterstain Brief H2O rinse Methylene Blue 30-45 Red sec. Blue