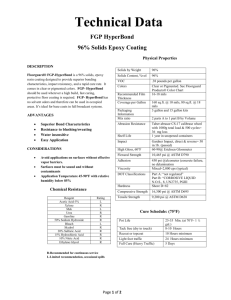
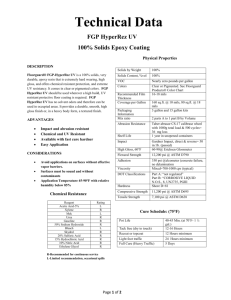
研究方法作業報告四
advertisement

研 究 方 法 作 業 報 告 四 光 電 工 程 研 究 所 中英文專利的搜尋 組別 : 第 五 組 學生 : M94L0102 謝 侑 融 M94L0207 楊 欽 堯 一、中華民國專利 先直接進入『智慧財產局』首頁(網址 : http://www.tipo.gov.tw/) 、點選『專利』欄位 進入之後、選擇『專利資料檢索』 進入之後、點選『中華民國專利公報檢索』 進入之後、勾選全部(如紅色圈起來)、然後在按確定 先由『簡易檢索』搜尋、在不限欄位裡輸入欲尋找的關鍵字『TiO2』 結果找到 1109 筆資料 由於筆數太大所以改輸入另一個關鍵字『photocatalyst』、年份不限、 輸入完之後再按『執行檢索』 、共找到 6 筆資料如下 : 第 1 筆 of 本國專利發明公開公報 專利類型 發明 公告/公開號 200536901 專利名稱 光觸媒之塗覆組成物=COATING COMPOSITION OF PHOTOCATALYST 專利影像 公告/公開日 2005/11/16 期 公報卷期 0322 申請日期 0940315 申請案號 094107908 國際分類號 C09D-001/11 發明人/地址 酒谷能彰 SAKATANI, YOSHIAKI 日本; /國家 酙口誠一 SAKAGUCHI, SEIICHI 日本 申請人/地址 住友化學股份有限公司 SUMITOMO CHEMICAL COMPANY, LIMITED /國家 日本; 朝日化學工業股份有限公司 ASAHI CHEMICAL COMPANY, LIMITED 日本 專利代理人 林志剛 摘要 一種光觸媒塗覆組成物,其包括光觸媒、烷氧化矽、鋯化合物、膠態矽石、 及液態介質,其中鋯化合物含量(以鋯原子計)為烷氧化矽莫耳含量(以矽原 子計)之 0.3 至 3 倍,該光觸媒塗覆組成物可形成具有高黏合力黏合至基材 的光觸媒塗覆膜。 專利範圍 一種光觸媒塗覆組成物,其包括光觸媒、烷氧化矽、鋯化合物、膠態矽石、 及液態介質,其中鋯化合物含量(以鋯原子計)為烷氧化矽莫耳含量(以矽原 子計)之 0.3 至 3 倍,該光觸媒塗覆組成物可形成具有高黏合力黏合至基材 的光觸媒塗覆膜。 第 2 筆 of 本國專利發明公開公報 專利類型 發明 公告/公開號 200530028 專利名稱 光觸媒片、其接合方法以及其製造方法 = PHOTOCATALYST SHEET, METHOD FOR ADHESION AND PRODUCING THE SAME 專利影像 公告/公開日 2005/09/16 期 公報卷期 0318 申請日期 0931214 申請案號 093138687 國際分類號 B32B-027/00 發明人/地址 豊田宏 TOYODA, HIROSHI 日本; /國家 阿部和廣 ABE, KAZUHIRO 日本; 中田貴之 NAKATA, TAKAYUKI 日本 申請人/地址 太陽工業股份有限公司 TAIYO KOGYO CORPORATION 日本 /國家 專利代理人 洪武雄 ; 陳昭誠 摘要 本發明係提供基材或光觸媒含有層之樹脂不會被光觸媒粒子分解、光觸媒 片之間容易接合、且可獲得光觸媒之光氧化還原效用之光觸媒片、其接合 方法及其製造方法。光觸媒片(1b)由纖維等基材(2)及包覆在基材(2)雙面 之覆膜層(3)所構成,覆膜層(3)係使經磷灰石包覆之光觸媒粒子(4)分散並 以樹脂加以固定而成之光觸媒含有層。此時,在光觸媒含有層表面之經磷 灰石包覆之光觸媒粒子(4),係以具有從光觸媒含有層表面露出之部分之形 式固定。將光觸媒片(1b)彼此接合時,係在不除去各光觸媒片(1b)之光觸媒 含有層之情況下使接合面互相重疊、熱熔著而接合。 專利範圍 本發明係提供基材或光觸媒含有層之樹脂不會被光觸媒粒子分解、光觸媒 片之間容易接合、且可獲得光觸媒之光氧化還原效用之光觸媒片、其接合 方法及其製造方法。光觸媒片(1b)由纖維等基材(2)及包覆在基材(2)雙面 之覆膜層(3)所構成,覆膜層(3)係使經磷灰石包覆之光觸媒粒子(4)分散並 以樹脂加以固定而成之光觸媒含有層。此時,在光觸媒含有層表面之經磷 灰石包覆之光觸媒粒子(4),係以具有從光觸媒含有層表面露出之部分之形 式固定。將光觸媒片(1b)彼此接合時,係在不除去各光觸媒片(1b)之光觸媒 含有層之情況下使接合面互相重疊、熱熔著而接合。 專利類型 新型 公告/公開號 M259809 專利名稱 以理化、生化衛材統合水療、洗淨及環保等多功能之電腦馬桶健康裝置 專利影像 公告/公開日 2005/03/21 期 公報卷期 3209 申請日期 0930428 申請案號 093206552 國際分類號 E03D-009/08 ; E03D-009/02 發明人/地址 李振邦 臺北市大安區安和路 1 段 90 巷 20 號 2 樓 /國家 申請人/地址 李振邦 臺北市大安區安和路 1 段 90 巷 20 號 2 樓; /國家 木股弘巳 日本 專利代理人 黃世興 臺北市中山區建國北路 3 段 82 號 2 樓 專利範圍 1.一種以理化、生化衛材統合水療、洗淨及環保等多功能之電腦馬桶健康 裝置,係包括: 一水療裝置連接一淨水裝置彼設於一機殼之中,該水療裝置主要為一水療 噴嘴架設於電腦馬桶座上俾將淨水噴射入用者之肛門與直腸中以沖洗糞 便、化解便秘者;以及 一淨氣裝置安裝於該機殼內,以吹出除臭、殺菌帶有負離子有益健康之淨 化空氣者。 2.如申請專利範圍第 1 項之以理化、生化衛材統合水療、洗淨及環保等多 功能之電腦馬桶健康裝置,其中該淨水裝置及該淨氣裝置,係分別藉電氣石 (tourmaline)、光觸媒( photocatalyst )及有效(或有用)微生物群(effective microorganisms)等理化、生化衛材以發揮除臭、殺菌、及淨化水質與空 氣,兼具保健與環保之多功能者。 3.如申請專利範圍第 1 項之電腦馬桶健康裝置,其中該淨水裝置係包括一 溫水箱其內置入該理化、生化衛材以淨化水質且提供溫水以沖洗人體腸 道、肛門者。 4.如申請專利範圍第 2 項之電腦馬桶健康裝置,其中該光觸媒係以二氧化 鈦製得,彼於光照射下會發生光觸媒作用分解病毒,以殺菌、淨化水質及空 氣者。 5.如申請專利範圍第 1 項之電腦馬桶健康裝置,其中該淨水裝置係設有導 管直接連通電腦馬桶座之前噴頭及後噴頭,以供應前噴洗、後噴洗所需之 淨化溫水流者。 6.如申請專利範圍第 1 項之電腦馬桶健康裝置,其中該水療裝置係包括: 一噴嘴;一輸水管連接該淨水裝置以輸入淨化溫水流者;一支架連設於一座 板上,該座板置於馬桶座上者,該支架用以撐連該輸水管之出口端以可拆卸 地插裝該噴嘴於該輸水管出口端上者。 7.如申請專利範圍第 6 項之電腦馬桶健康裝置,其中該座板藉底部所連設 之至少一對吸盤吸附於馬桶座或瓷座上者。 8.如申請專利範圍第 6 項之電腦馬桶健康裝置,其中該噴嘴製成一長管,於 頂部中央開設一主噴孔,向上噴水入人體直腸之中;於鄰頂部之水管周圍開 設多個下斜噴孔,以側向噴出水流稍微向下以沖洗便物沿著肛門以重力方 向下流進入馬桶之中者。 9.如申請專利範圍第 6 項之電腦馬桶健康裝置,其中該噴嘴係製作為可拋 棄型(disposable),用完就丟,以維衛生者。 10.如申請專利範圍第 1 項之電腦馬桶健康裝置,其中該淨氣裝置係包括: 紫外光燈或加熱燈、風扇、網具等,係設於該機殼內,該網具內及機殼內係 裝入前述之理化、生化衛材含有電氣石、光觸媒及有效微生物群者,令空 氣進入機殼后,透過此等衛材將空氣淨化、除臭,並釋出有益於人體之負離 子及遠紅外線,由設於該機殼上之出風口吹出至馬桶座所處之浴廁內,以提 供一除臭、淨化、環保、健康之清新空氣者。 圖式簡單說明: 第 1 圖係本新型之斜視示意圖。 第 2 圖係本新型之水療裝置示意圖。 第 3 圖係該噴嘴之示意圖。 第 4 圖係本新型之調控裝置示意圖。 第 4 筆 of 本國專利發明公開公報 專利類型 發明 公告/公開號 200507936 專利名稱 光觸媒塗佈液、光觸媒膜及光觸媒構件= PHOTOCATALYST COATING SOLUTION, PHOTOCATALYST FILM AND PHOTOCATALYST MEMBER 專利影像 公告/公開日 2005/03/01 期 公報卷期 0305 申請日期 0930429 申請案號 093112043 國際分類號 B01J-035/02 ; C09D-185/00 發明人/地址 田中尚樹 TANAKA, NAOKI 日本; /國家 鈴木宏和 SUZUKI, HIROKAZU 日本; 小池匡 KOIKE, TADASHI 日本 申請人/地址 宇部日東化成股份有限公司 UBE-NITTO KASEI CO., LTD. 日本 /國家 專利代理人 林志剛 摘要 發明係關於超親水性、暗處的超親水維持性能等優異的光觸媒功能,且可 於有機基材上形成具良好耐久性的光觸媒膜,且安定性優異之光觸媒塗佈 液,以及使用其形成之光觸媒膜;提供包合(A)銳鈦礦型結晶構成之氧化鈦 微粒子;(B)膠體二氧化矽;以及(C)鈦烷氧化物;以加水分解.縮合物構成的 黏合劑,且基於固體成分全體的量,(A)成分含有量為 5-50 質量%(B)成分含 有量,其固體成分為 25-75 質量%,以及(C)成分含有量,以 TiO2,換算其固體 成分為 10-55 質量%之光觸媒塗佈液,以及使用該塗佈液形成的光觸媒膜。 專利範圍 發明係關於超親水性、暗處的超親水維持性能等優異的光觸媒功能,且可 於有機基材上形成具良好耐久性的光觸媒膜,且安定性優異之光觸媒塗佈 液,以及使用其形成之光觸媒膜;提供包合(A)銳鈦礦型結晶構成之氧化鈦 微粒子;(B)膠體二氧化矽;以及(C)鈦烷氧化物;以加水分解.縮合物構成的 黏合劑,且基於固體成分全體的量,(A)成分含有量為 5-50 質量%(B)成分含 有量,其固體成分為 25-75 質量%,以及(C)成分含有量,以 TiO2,換算其固體 成分為 10-55 質量%之光觸媒塗佈液,以及使用該塗佈液形成的光觸媒膜。 第 5 筆 of 本國專利公報 (民國 89-迄今) 專利類型 發明 公告/公開號 00586923 專利名稱 具有光催化作用塗層的人工水晶體 專利影像 公告/公開日 2004/05/11 期 公報卷期 3114 申請日期 2002/10/25 申請案號 091125130 國際分類號 A61F-002/14 發明人/地址 蔡明霖 臺北市文山區興隆路四段一四五巷六十四號三樓 中華民國; /國家 鄭盛文 新竹市建中一路三十七號十七樓之五 中華民國; 郭宗南 新竹市光復路二段二九八巷九弄十七號四樓 中華民國; 彭素珍 臺北市中正區永春街一五四巷四弄三十九號 中華民國 申請人/地址 蔡明霖 臺北市文山區興隆路四段一四五巷六十四號三樓 中華民國; /國家 鄭盛文 新竹市建中一路三十七號十七樓之五 中華民國; 郭宗南 新竹市光復路二段二九八巷九弄十七號四樓 中華民國; 彭素珍 臺北市中正區永春街一五四巷四弄三十九號 中華民國 專利代理人 惲軼群 臺北市松山區南京東路三段二四八號七樓; 陳文郎 臺北市松山區南京東路三段二四八號七樓 摘要 本發明揭示一種人工水晶體,其中該人工水晶體的光 學水晶體本體的至少一個表面的至少一部份被塗覆以一由 一光觸媒( photocatalyst )所構成的光催化作用塗層,藉此, 在一光束通經該光學水晶體本體時,該光催化作用塗層被 活化而釋出自由基氧化分解沾附於光學人工水晶體本體表 面的微生物與水晶體上皮細胞,降低白內障病人手術後併 發症[諸如眼內炎(endophthalmitis)與續發性白內障 (after-cataract)]的發生率。 專利範圍 1.一種人工水晶體,其包含: 一光學水晶體本體(optic lens body),其具有一前表面與一後表面; 一從該光學水晶體本體側向延伸而出的水晶體置放構件,用以將該光學水 晶體本體安置在一使用者的一個眼球內;以及 一光催化作用塗層,其係由一光觸媒所構成,且被塗覆在該光學水晶體本體 的前表面與後表面之至少一者的至少一部份上,藉此,在一光束通經該光學 水晶體本體時,該光催化作用塗層被活化而釋出會展現抗微生物效用之自 由基。2.如申請專利範圍第 1 項之人工水晶體,其中該光催化作用塗層被 塗覆在該光學水晶體本體之前表面上。3.如申請專利範圍第 2 項之人工水 晶體,其中該光催化作用塗層被塗覆在該光學水晶體本體之前表面的一個 會接觸一使用者的一眼的眼前房之區域上。4.如申請專利範圍第 1 項之人 工水晶體,其中該光催化作用塗層被塗覆在該光學水晶體本體之後表面 上。5.如申請專利範圍第 4 項之人工水晶體,其中該光催化作用塗層被塗 覆在該光學水晶體本體之後表面的一個會接觸一使用者的一眼的水晶體 囊袋之區域上。6.如申請專利範圍第 1 項之人工水晶體,其中該光催化作 用塗層被塗覆在該光學水晶體本體之前表面與後表面上。7.如申請專利範 圍第 1 項之人工水晶體,其中該光觸媒係擇自於下列群組:一種鈦氧化物、 ZnO、SnO2.WO3.CaTiO3.MoO3.NbO5.KNbO3.SrTiO3.CdSe、 Fe2O3.Ta2O5.Tix(Zrl-x)O2(其中 x=0 或 1)以及 CdS。8.如申請專利範圍 第 7 項之人工水晶體,其中該光觸媒係為一擇自於下列群組中的鈦氧化物: 一氧化鈦、二氧化鈦、三氧化二鈦以及五氧化三鈦。9.如申請專利範圍第 8 項之人工水晶體,其中該光觸媒係為二氧化鈦(TiO2)。10.如申請專利範 圍第 1 項之人工水晶體,其中該光催化作用塗層具有一厚度係介於 2nm-200nm 之範圍內。11.如申請專利範圍第 1 項之人工水晶體,其中該 光學水晶體本體係由一擇自於下列群組中的物質所製成:聚甲基丙烯酸甲 酯、矽酮聚合物或丙烯酸聚合物。12.如申請專利範圍第 1 項之人工水晶 體,其中該水晶體置放構件與該光學水晶體本體是一體成型製造的。13.如 申請專利範圍第 1 項之人工水晶體,其中該水晶體置放構件與該光學水晶 體本體是分開製造的。14.如申請專利範圍第 1 項之人工水晶體,其中該水 晶體置放構件包括兩個腳架,該等腳架各有一端部被側向地連接於該光學 水晶體本體上。15.如申請專利範圍第 14 項之人工水晶體,其中該腳架係 以一擇自於下列群組的物質所製成:聚甲基丙烯酸甲酯、聚丙烯或聚偏氟 乙烯。16.一種用以製造一人工水晶體之方法,其包含下列步驟: (a)提供一人工水晶體,該人工水晶體包含有:一具有一前表面與一後表面 之光學水晶體本體;以及一從該光學水晶體本體側向延伸而出的水晶體置 放構件,用以將該光學水晶體本體安置在一使用者的一個眼球內;以及 (b)將一光觸媒塗覆於該光學水晶體本體的至少一個表面的至少一部份上 而形成一光催化作用塗層。17.如申請專利範圍第 16 項之方法,其中步驟 (b)是藉由至少一種選自於下列之方式來進行:蒸鍍法、濺鍍法、化學氣相 沉積法、電漿化學氣相沉積法以及物理氣相沉積法。18.如申請專利範圍 第 16 項之方法,其中在步驟(b)中,該光催化作用塗層被形成在該光學水晶 體本體之前表面上。19.如申請專利範圍第 18 項之方法,其中在步驟(b)後, 該光催化作用塗層被形成在該光學水晶體本體之前表面的一個會接觸一 使用者的一眼的眼前房之區域上。20.如申請專利範圍第 16 項之方法,其 中在步驟(b)後,該光催化作用塗層被形成在該光學水晶體本體之後表面 上。21.如申請專利範圍第 20 項之方法,其中在步驟(b)後,該光催化作用塗 層被形成在該光學水晶體本體之後表面的一個會接觸一使用者的一眼的 水晶體囊袋之區域上。22.如申請專利範圍第 16 項之方法,其中在步驟(b) 後,該光催化作用塗層被形成在該光學水晶體本體之前表面與後表面上。 23.如申請專利範圍第 16 項之方法,其中步驟(b)所用的光觸媒係擇自於下 列群組:一種鈦氧化物、ZnO、 SnO2.WO3.CaTiO3.MoO3.NbO5.KNbO3.SrTiO3.CdSe、 Fe2O3.Ta2O5.Tix(Zrl-x)O2(其中 x=0 或 1)以及 CdS。24.如申請專利範 圍第 23 項之方法,其中步驟(b)所用的光觸媒係為一擇自於下列群組中之 鈦氧化物:一氧化鈦、二氧化鈦、三氧化二鈦或五氧化三鈦。25.如申請專 利範圍第 24 項之方法,其中步驟(b)所用的光觸媒係為二氧化鈦(TiO2)。26. 如申請專利範圍第 16 項之方法,其中在步驟(b)之後,一具有一厚度範圍在 2nm-200nm 之間的光催化作用塗層被形成。27.如申請專利範圍第 16 項 之方法,其中該水晶體本體係以一擇自於下列群組中的物質所製成:聚甲基 丙烯酸甲酯、矽酮聚合物或丙烯酸聚合物。28.如申請專利範圍第 16 項之 方法,其中在步驟(a)提供的人工水晶體中,該水晶體置放構件與該光學水 晶體本體是一體成型製造的。29.如申請專利範圍第 16 項之方法,其中在 步驟(a)提供的人工水晶體中,該水晶體置放構件與該光學水晶體本體是分 開製造的。30.如申請專利範圍第 16 項之方法,其中在步驟(a)提供的人工 水晶體中,該水晶體置放構件包括兩個腳架,該等腳架各有一端部被側向地 連接於該光學水晶體本體上。31.如申請專利範圍第 30 項之方法,其中該 腳架係以一擇自於下列群組的物質所製成:聚甲基丙烯酸甲酯、聚丙烯或 聚偏氟乙烯。圖式簡單說明: 第 1 圖是一示意平面圖,其中顯示一個適用於本發明的人工水晶體的結構; 第 2 圖為第 1 圖中所示的人工水晶體的一個示意剖面圖; 第 3 圖是一示意透視圖,其中顯示另一種適用於本發明的可摺疊式平板狀 (plate-type)人工水晶體的結構; 第 4 圖為一剖視圖,其顯示在一光學水晶體本體之前表面與後表面上各形 成有一光催化作用塗層; 第 5 圖為沿第 4 圖所示虛線所取區域之一部分放大的剖視圖,其顯示有一 光催化作用塗層被形成在一光學水晶體本體的前表面上; 第 6A 圖為一示意平面圖,其顯示一於前表面上形成有一光催化作用塗層 的人工水晶體被置於一眼球內,並有一光束通經該人工水晶體的光學水晶 體本體前表面之情形; 第 6B 圖為一示意平面圖,其顯示一於後表面上形成有一光催化作用塗層 的人工水晶體被置於一眼球內,並有一光束通經該人工水晶體的光學水晶 體本體後表面之情形; 第 7A 圖為一示意透視圖,其顯示將帶有由 TiO2 所構成的光催化作用塗層 的光學水晶體本體測試樣品置放於一試驗盤的井孔內,以進行細菌或細胞 生長抑制試驗; 第 7B 圖為一試驗盤井孔之放大剖面圖,其顯示一光學水晶體本體測試樣 品被放在一試驗盤的一個井孔內,且被覆蓋以一細胞懸浮液,再以 UV 光予 以照射處理之示意圖; 第 8 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗層 的人工水晶體對於大腸桿菌的分解效果,其中以 MDA 數值作為測定指標; 第 9 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗層 的人工水晶體對於大腸桿菌存活率的影響; 第 10 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗 層的人工水晶體對於人類水晶體上皮細胞生長之分解效果,其中以 MDA 數值作為測定指標;以及 第 11 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗 層的人工水晶體對於人類水晶體上皮細胞菌存活率之影響。 第 6 筆 of 本國專利公報 (民國 89-迄今) 專利類型 發明 公告/公開號 00452605 專利名稱 紫外線遮蔽材料及其製造方法 專利影像 公告/公開日 2001/09/01 期 公報卷期 2825 申請日期 1998/10/08 申請案號 087116667 國際分類號 C23C-020/06 發明人/地址 松田泰宏 日本 日本 /國家 申請人/地址 日揮化學股份有限公司 日本 日本 /國家 專利代理人 陳燦暉 台北巿城中區武昌街一段六十四號八樓; 洪武雄 台北巿城中區武昌街一段六十四號八樓; 陳昭誠 台北巿武昌街一段六十四號八樓 摘要 本發明係有關紫外線遮蔽材料及其製造方法者。其目 的在提供一種不使由氧化鈦、氧化鈦水合物等或將這些物 質擔持於表面上之無機物質所構成之紫外線遮蔽機能物質 表現所具有的光催化劑( photocatalyst )機能,不使經擔 持及分散之有機基材光老化,而能夠僅表現紫外線遮蔽機 能的紫外線遮蔽材料,以及以黏土礦物包覆紫外線遮蔽機 能物質之方法。詳言之,係以將紫外線遮蔽機能物質以黏 土礦物包覆為其特徵之將紫外線遮蔽材料及紫外線遮蔽機 能物質以黏土礦物包覆而成之紫外線遮蔽材料的製造方法 ,該製造方法以包括 a)使黏土礦物分散於溶劑中之步驟、 b)使紫外線遮蔽機能物質分散於溶劑中之步驟、 c)將前述步驟 a)及 b)中製得之 2 種分散液混合並包覆之步 驟、 d)使前述混合物進行固液分離之步驟、 e)將前述步驟 d)製得之固形物加熱之步驟、 為其特徵之使紫外線遮蔽機能物質以黏土機能物質以黏土 礦物包覆之方法。 專利範圍 1.一種紫外線遮蔽材料,係以將紫外線遮蔽機能物質以黏土礦物加以包覆 為其特徵者,其中紫外線遮蔽機能物質於該紫外線遮蔽材料中之含量為 16 至 83 重量%。2.如申請專利範圍第 1 項之紫外線遮蔽材料,其中該點土礦 物為頁狀矽酸鹄(phillosilicate)者。3.如申請專利範圍第 2 項之紫外線遮蔽 材料,其中該頁狀矽酸鹄為綠多水高嶺土(smectite)、蛭石(vermiculite)、以 及雲母(mica)者。4.如申請專利範圍第 1 至 3 項中任一項之紫外線遮蔽材 料,其中該紫外線遮蔽機能物質係送自氧化鈦、氧化鈦水合物、擔持有氧 化鈦及/或氧鈦水合物之無機物質中至少一種者。5.一種紫外線遮蔽材料之 製造方法,該方法包括下列步驟: a)使黏土礦物分散於溶劑中之步驟、 b)使紫外線遮蔽機能物質分散於溶劑中之步驟、 c)將前述步驟 a)及 b)製得之 2 種分散液混合並包覆之步驟、 d)使前述混合物進行固液分離之步驟、 e)將前述步驟 d)製得之固形物加熱之步驟。6.如申請專利範圍第 5 項之紫 外線遮蔽材料製造方法,其中該步驟 e)之加熱係在 60℃以上 350℃以下之 溫度中加熱到在 110℃乾燥減量為 3 至 15wt%範圍者。7.如申請專利範圍 第 5 項之紫外線遮蔽材料之製造方法,其中該黏土礦物係頁狀矽酸鹄者。 8.如申請專利範圍第 7 項之紫外線遮蔽材料之製造方法,其中該頁狀矽酸 鹄係綠多水高嶺土、蛭石、及雲母者。9.如申請專利範圍第 5 至 8 項中任 一項之紫外線遮蔽材料之製造方法,其中該紫外線遮蔽機能物質係選自氧 化鈦、氧化鈦水合物、擔持有氧化鈦及/或氧化鈦水合物之無機物質中至 少一種者。圖式簡單說明: 第一圖分別表示實施例 1(a)及比較例 1(b)中製得粉體之 UV 反射光譜。 第二圖表示實施例 1 及比較例 2 中製得粉體之膨潤及分散前後的粒度分 布。 第三圖表示實施例 2 及比較例 3.4 中製得粉體之作為光催化劑機能之氧化 分解性能。 雜項資料 勘誤表(第 28 卷第 33 期) 原 2833.25.txt 始 資 料 更 更 正第 087116667 號 專利案 之 申請 專利 範圍 : 正 專 利 原 (原 刊於第 28 卷 第 25 期第 452605 號 公告 ) 刊 來 源 正 申 請 專利 範圍 : 2.如申 請專 利範 圍第 1 項 之紫 外 線遮 蔽材 料 ,其中 該點 土礦 物 為 頁 狀 矽酸 鹄(phillosilicate)者。 4.如申 請專 利範 圍第 1 至 3 項 中 任一 項之 紫外 線遮 蔽材料 ,其中 該 紫 外 線遮 蔽機 能物 質係 送自氧 化 鈦、氧化 鈦水 合物、擔持 有 氧化 鈦 及 /或 氧鈦 水合 物之 無機 物質 中 至少 一種 者。 誤 申 請 專利 範圍 : 2.如申 請專 利範 圍第 1 項 之紫 外 線遮 蔽 材 料,其中 該黏 土礦 物 為 頁 狀 矽酸 鹄(phillosilicate)者。 4.如申 請專 利範 圍第 1 至 3 項 中 任一 項之 紫外 線遮 蔽材料 ,其中 該 紫 外 線遮 蔽機 能物 質係 選自氧 化 鈦、氧化 鈦水 合物、擔持 有 氧化 鈦 及 /或 氧鈦 水合 物之 無機 物質 中 至少 一種 者。 接下來改用『進階搜尋』來搜尋看看、輸入關鍵字『TiO2 and photocatalyst』 總共找到 2 筆資料 第 1 筆 of 本國專利發明公開公報 專利類型 發明 公告/公開號 200507936 專利名稱 光觸媒塗佈液、光觸媒膜及光觸媒構件= PHOTOCATALYST COATING SOLUTION, PHOTOCATALYST FILM AND PHOTOCATALYST MEMBER 專利影像 公告/公開日 2005/03/01 期 公報卷期 0305 申請日期 0930429 申請案號 093112043 國際分類號 B01J-035/02 ; C09D-185/00 發明人/地址 田中尚樹 TANAKA, NAOKI 日本; /國家 鈴木宏和 SUZUKI, HIROKAZU 日本; 小池匡 KOIKE, TADASHI 日本 申請人/地址 宇部日東化成股份有限公司 UBE-NITTO KASEI CO., LTD. 日本 /國家 專利代理人 林志剛 摘要 發明係關於超親水性、暗處的超親水維持性能等優異的光觸媒功能,且可 於有機基材上形成具良好耐久性的光觸媒膜,且安定性優異之光觸媒塗佈 液,以及使用其形成之光觸媒膜;提供包合(A)銳鈦礦型結晶構成之氧化鈦 微粒子;(B)膠體二氧化矽;以及(C)鈦烷氧化物;以加水分解.縮合物構成的 黏合劑,且基於固體成分全體的量,(A)成分含有量為 5-50 質量%(B)成分含 有量,其固體成分為 25-75 質量%,以及(C)成分含有量,以 TiO2 ,換算其固 體成分為 10-55 質量%之光觸媒塗佈液,以及使用該塗佈液形成的光觸媒 膜。 專利範圍 發明係關於超親水性、暗處的超親水維持性能等優異的光觸媒功能,且可 於有機基材上形成具良好耐久性的光觸媒膜,且安定性優異之光觸媒塗佈 液,以及使用其形成之光觸媒膜;提供包合(A)銳鈦礦型結晶構成之氧化鈦 微粒子;(B)膠體二氧化矽;以及(C)鈦烷氧化物;以加水分解.縮合物構成的 黏合劑,且基於固體成分全體的量,(A)成分含有量為 5-50 質量%(B)成分含 有量,其固體成分為 25-75 質量%,以及(C)成分含有量,以 TiO2 ,換算其固 體成分為 10-55 質量%之光觸媒塗佈液,以及使用該塗佈液形成的光觸媒 膜。 第 2 筆 of 本國專利公報 (民國 89-迄今) 專利類型 發明 公告/公開號 00586923 專利名稱 具有光催化作用塗層的人工水晶體 專利影像 公告/公開日 2004/05/11 期 公報卷期 3114 申請日期 2002/10/25 申請案號 091125130 國際分類號 A61F-002/14 發明人/地址 蔡明霖 臺北市文山區興隆路四段一四五巷六十四號三樓 中華民國; /國家 鄭盛文 新竹市建中一路三十七號十七樓之五 中華民國; 郭宗南 新竹市光復路二段二九八巷九弄十七號四樓 中華民國; 彭素珍 臺北市中正區永春街一五四巷四弄三十九號 中華民國 申請人/地址 蔡明霖 臺北市文山區興隆路四段一四五巷六十四號三樓 中華民國; /國家 鄭盛文 新竹市建中一路三十七號十七樓之五 中華民國; 郭宗南 新竹市光復路二段二九八巷九弄十七號四樓 中華民國; 彭素珍 臺北市中正區永春街一五四巷四弄三十九號 中華民國 專利代理人 惲軼群 臺北市松山區南京東路三段二四八號七樓; 陳文郎 臺北市松山區南京東路三段二四八號七樓 摘要 本發明揭示一種人工水晶體,其中該人工水晶體的光 學水晶體本體的至少一個表面的至少一部份被塗覆以一由 一光觸媒( photocatalyst )所構成的光催化作用塗層,藉此, 在一光束通經該光學水晶體本體時,該光催化作用塗層被 活化而釋出自由基氧化分解沾附於光學人工水晶體本體表 面的微生物與水晶體上皮細胞,降低白內障病人手術後併 發症[諸如眼內炎(endophthalmitis)與續發性白內障 (after-cataract)]的發生率。 專利範圍 1.一種人工水晶體,其包含: 一光學水晶體本體(optic lens body),其具有一前表面與一後表面; 一從該光學水晶體本體側向延伸而出的水晶體置放構件,用以將該光學水 晶體本體安置在一使用者的一個眼球內;以及 一光催化作用塗層,其係由一光觸媒所構成,且被塗覆在該光學水晶體本體 的前表面與後表面之至少一者的至少一部份上,藉此,在一光束通經該光學 水晶體本體時,該光催化作用塗層被活化而釋出會展現抗微生物效用之自 由基。2.如申請專利範圍第 1 項之人工水晶體,其中該光催化作用塗層被 塗覆在該光學水晶體本體之前表面上。3.如申請專利範圍第 2 項之人工水 晶體,其中該光催化作用塗層被塗覆在該光學水晶體本體之前表面的一個 會接觸一使用者的一眼的眼前房之區域上。4.如申請專利範圍第 1 項之人 工水晶體,其中該光催化作用塗層被塗覆在該光學水晶體本體之後表面 上。5.如申請專利範圍第 4 項之人工水晶體,其中該光催化作用塗層被塗 覆在該光學水晶體本體之後表面的一個會接觸一使用者的一眼的水晶體 囊袋之區域上。6.如申請專利範圍第 1 項之人工水晶體,其中該光催化作 用塗層被塗覆在該光學水晶體本體之前表面與後表面上。7.如申請專利範 圍第 1 項之人工水晶體,其中該光觸媒係擇自於下列群組:一種鈦氧化物、 ZnO、SnO2.WO3.CaTiO3.MoO3.NbO5.KNbO3.SrTiO3.CdSe、 Fe2O3.Ta2O5.Tix(Zrl-x)O2(其中 x=0 或 1)以及 CdS。8.如申請專利範圍 第 7 項之人工水晶體,其中該光觸媒係為一擇自於下列群組中的鈦氧化物: 一氧化鈦、二氧化鈦、三氧化二鈦以及五氧化三鈦。9.如申請專利範圍第 8 項之人工水晶體,其中該光觸媒係為二氧化鈦( TiO2 )。10.如申請專利範 圍第 1 項之人工水晶體,其中該光催化作用塗層具有一厚度係介於 2nm-200nm 之範圍內。11.如申請專利範圍第 1 項之人工水晶體,其中該 光學水晶體本體係由一擇自於下列群組中的物質所製成:聚甲基丙烯酸甲 酯、矽酮聚合物或丙烯酸聚合物。12.如申請專利範圍第 1 項之人工水晶 體,其中該水晶體置放構件與該光學水晶體本體是一體成型製造的。13.如 申請專利範圍第 1 項之人工水晶體,其中該水晶體置放構件與該光學水晶 體本體是分開製造的。14.如申請專利範圍第 1 項之人工水晶體,其中該水 晶體置放構件包括兩個腳架,該等腳架各有一端部被側向地連接於該光學 水晶體本體上。15.如申請專利範圍第 14 項之人工水晶體,其中該腳架係 以一擇自於下列群組的物質所製成:聚甲基丙烯酸甲酯、聚丙烯或聚偏氟 乙烯。16.一種用以製造一人工水晶體之方法,其包含下列步驟: (a)提供一人工水晶體,該人工水晶體包含有:一具有一前表面與一後表面 之光學水晶體本體;以及一從該光學水晶體本體側向延伸而出的水晶體置 放構件,用以將該光學水晶體本體安置在一使用者的一個眼球內;以及 (b)將一光觸媒塗覆於該光學水晶體本體的至少一個表面的至少一部份上 而形成一光催化作用塗層。17.如申請專利範圍第 16 項之方法,其中步驟 (b)是藉由至少一種選自於下列之方式來進行:蒸鍍法、濺鍍法、化學氣相 沉積法、電漿化學氣相沉積法以及物理氣相沉積法。18.如申請專利範圍 第 16 項之方法,其中在步驟(b)中,該光催化作用塗層被形成在該光學水晶 體本體之前表面上。19.如申請專利範圍第 18 項之方法,其中在步驟(b)後, 該光催化作用塗層被形成在該光學水晶體本體之前表面的一個會接觸一 使用者的一眼的眼前房之區域上。20.如申請專利範圍第 16 項之方法,其 中在步驟(b)後,該光催化作用塗層被形成在該光學水晶體本體之後表面 上。21.如申請專利範圍第 20 項之方法,其中在步驟(b)後,該光催化作用塗 層被形成在該光學水晶體本體之後表面的一個會接觸一使用者的一眼的 水晶體囊袋之區域上。22.如申請專利範圍第 16 項之方法,其中在步驟(b) 後,該光催化作用塗層被形成在該光學水晶體本體之前表面與後表面上。 23.如申請專利範圍第 16 項之方法,其中步驟(b)所用的光觸媒係擇自於下 列群組:一種鈦氧化物、ZnO、 SnO2.WO3.CaTiO3.MoO3.NbO5.KNbO3.SrTiO3.CdSe、 Fe2O3.Ta2O5.Tix(Zrl-x)O2(其中 x=0 或 1)以及 CdS。24.如申請專利範 圍第 23 項之方法,其中步驟(b)所用的光觸媒係為一擇自於下列群組中之 鈦氧化物:一氧化鈦、二氧化鈦、三氧化二鈦或五氧化三鈦。25.如申請專 利範圍第 24 項之方法,其中步驟(b)所用的光觸媒係為二氧化鈦( TiO2 )。 26.如申請專利範圍第 16 項之方法,其中在步驟(b)之後,一具有一厚度範圍 在 2nm-200nm 之間的光催化作用塗層被形成。27.如申請專利範圍第 16 項之方法,其中該水晶體本體係以一擇自於下列群組中的物質所製成:聚甲 基丙烯酸甲酯、矽酮聚合物或丙烯酸聚合物。28.如申請專利範圍第 16 項 之方法,其中在步驟(a)提供的人工水晶體中,該水晶體置放構件與該光學 水晶體本體是一體成型製造的。29.如申請專利範圍第 16 項之方法,其中 在步驟(a)提供的人工水晶體中,該水晶體置放構件與該光學水晶體本體是 分開製造的。30.如申請專利範圍第 16 項之方法,其中在步驟(a)提供的人 工水晶體中,該水晶體置放構件包括兩個腳架,該等腳架各有一端部被側向 地連接於該光學水晶體本體上。31.如申請專利範圍第 30 項之方法,其中 該腳架係以一擇自於下列群組的物質所製成:聚甲基丙烯酸甲酯、聚丙烯 或聚偏氟乙烯。圖式簡單說明: 第 1 圖是一示意平面圖,其中顯示一個適用於本發明的人工水晶體的結構; 第 2 圖為第 1 圖中所示的人工水晶體的一個示意剖面圖; 第 3 圖是一示意透視圖,其中顯示另一種適用於本發明的可摺疊式平板狀 (plate-type)人工水晶體的結構; 第 4 圖為一剖視圖,其顯示在一光學水晶體本體之前表面與後表面上各形 成有一光催化作用塗層; 第 5 圖為沿第 4 圖所示虛線所取區域之一部分放大的剖視圖,其顯示有一 光催化作用塗層被形成在一光學水晶體本體的前表面上; 第 6A 圖為一示意平面圖,其顯示一於前表面上形成有一光催化作用塗層 的人工水晶體被置於一眼球內,並有一光束通經該人工水晶體的光學水晶 體本體前表面之情形; 第 6B 圖為一示意平面圖,其顯示一於後表面上形成有一光催化作用塗層 的人工水晶體被置於一眼球內,並有一光束通經該人工水晶體的光學水晶 體本體後表面之情形; 第 7A 圖為一示意透視圖,其顯示將帶有由 TiO2 所構成的光催化作用塗 層的光學水晶體本體測試樣品置放於一試驗盤的井孔內,以進行細菌或細 胞生長抑制試驗; 第 7B 圖為一試驗盤井孔之放大剖面圖,其顯示一光學水晶體本體測試樣 品被放在一試驗盤的一個井孔內,且被覆蓋以一細胞懸浮液,再以 UV 光予 以照射處理之示意圖; 第 8 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗 層的人工水晶體對於大腸桿菌的分解效果,其中以 MDA 數值作為測定指 標; 第 9 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用塗 層的人工水晶體對於大腸桿菌存活率的影響; 第 10 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用 塗層的人工水晶體對於人類水晶體上皮細胞生長之分解效果,其中以 MDA 數值作為測定指標;以及 第 11 圖為一柱狀圖,其顯示帶有與未帶有由 TiO2 所構成的光催化作用 塗層的人工水晶體對於人類水晶體上皮細胞菌存活率之影響。 二、美國專利 先進入美國專利商標局網站(http://www.uspto.gov/)、進入之後點選 『Search』、之後再選擇『Quick Search』 、進入頁面如下 : 輸入關鍵字『TiO2 and photocatalyst』結果共找到 74 筆資料、由於太 多筆數、所以改用其他的關鍵字搜尋。 再輸入關鍵字『TiO2 thin film』、 『photocatalyst』 總共找到 4 筆資料 點選第一筆專利號碼、詳細內容如下 : (1 United States Patent Boire , of 4) 6,846,556 et al. January 25, 2005 Substrate with a photocatalytic coating Abstract The subject of the invention is a glass-, ceramic- or vitroceramic-based substrate (1) provided on at least part of at least one of its faces with a coating (3) with a photocatalytic property containing at least partially crystalline titanium oxide. It also relates to the applications of such a substrate and to its method of preparation. Inventors: Boire; Philippe (Paris, FR); Talpaert; Xavier (Paris, FR) Assignee: Saint-Gobain Glass France (Paris, FR) Appl. No.: 116164 Filed: April 5, 2002 428/325; 359/580; 359/582; 359/585; 359/586; 427/164; 427/165; 427/167; 428/432; 428/701; 428/702 Current U.S. Class: B32B 017/06; C23C016/00 Intern'l Class: 428/325,428,432,701,702 427/164,165,167,255 Field of Search: 359/580,582,585,586 References Cited [Referenced By] U.S. Patent Documents 3630796 Dec., 1971 Yokozawa et al. 4017661 Apr., 1977 Gillery. 4112142 Sep., 1978 Schroder et al. 4238276 Dec., 1980 Kinugawa et al. 4485146 Nov., 1984 Mizuhashi et al. 4544470 Oct., 1985 Hetrick. 4888038 Dec., 1989 Herrington et al. 4892712 Jan., 1990 Robertson et al. 5028568 Jul., 1991 Anderson et al. 5032241 Jul., 1991 Robertson et al. 5035784 Jul., 1991 Anderson et al. 5304394 Apr., 1994 Sauvinet et al. 5308805 May., 1994 Baker et al. 5342676 Aug., 1994 Zagdoun. 5348805 Sep., 1994 Zagdoun et al. 5368892 Nov., 1994 Berquier. 5389427 Feb., 1995 Berquier. 5478783 Dec., 1995 Higby et al. 5505989 Apr., 1996 Jenkinson. 5514454 May., 1996 Boire et al. 5525406 Jun., 1996 Goodman et al. 5547823 Aug., 1996 Murasawa et al. 5580364 Dec., 1996 Goodman et al. 5616532 Apr., 1997 Heller et al. 5618579 Apr., 1997 Boire et al. 5698262 Dec., 1997 Soubeyrand et al. 5735922 Apr., 1998 Woodward et al. 5745291 Apr., 1998 Jenkinson. 5749931 May., 1998 Goodman et al. 5751484 May., 1998 Goodman et al. 5755845 May., 1998 Woodward et al. 5764415 Jun., 1998 Nelson et al. 5773086 Jun., 1998 McCurdy et al. 5853866 Dec., 1998 Watanabe et al. 5854169 Dec., 1998 Heller et al. 5869187 Feb., 1999 Nakamura et al. 5939201 Aug., 1999 Boire et al. 5961843 Oct., 1999 Hayakawa et al. 6001462 Dec., 1999 Purvis et al. 6013372 Jan., 2000 Hayakawa et al. 6027766 Feb., 2000 Greenberg et al. 6027797 Feb., 2000 Watanabe et al. 6037289 Mar., 2000 Chopin et al. 6045896 Apr., 2000 Boire et al. 6054227 Apr., 2000 Greenberg et al. 6068914 May., 2000 Boire et al. 6090489 Jul., 2000 Hayakawa et al. 6103360 Aug., 2000 Caldwell et al. 6103363 Aug., 2000 Boire et al. 6106955 Aug., 2000 Ogawa et al. 6210779 Apr., 2001 Watanabe et al. 6228480 May., 2001 Kimura et al. 6238738 May., 2001 McCurdy. 6322881 Nov., 2001 Boire et al. 6326079 Dec., 2001 Philippe et al. 6387844 May., 2002 Fujishima et al. Foreign Patent Documents 0 544 577 Jun., 1993 EP. 0 581 216 Feb., 1994 EP. 2 320 499 Jun., 1998 GB. 2 320503 Jun., 1998 GB. 2 324098 Oct., 1998 GB. 2 355 273 Apr., 2001 GB. 5-3149281 Dec., 1978 JP. 63-100042 May., 1988 JP. 5-302173 Nov., 1993 JP. 7-99425 Mar., 1995 JP. 7-117600 Apr., 1995 JP. 7-182019 Jun., 1995 JP. 7-182020 Jun., 1995 JP. 7-205019 Jul., 1995 JP. 8-119673 May., 1996 JP. 8-164334 Jun., 1996 JP. 8-313705 Nov., 1996 JP. 9-173783 Jul., 1997 JP. 9-227157 Sep., 1997 JP. 9-227158 Sep., 1997 JP. 9-235140 Sep., 1997 JP. 9-241037 Feb., 1999 JP. WO 96/13327 May., 1996 WO. WO 96/29375 Sep., 1996 WO. WO 97/07069 Feb., 1997 WO. WO 97/10186 Mar., 1997 WO. WO 98/06510 Feb., 1998 WO. WO 98/08675 Feb., 1998 WO. WO 00/75087 Dec., 2000 WO. 428/325. WO 01/10790 Feb., 2001 WO. WO 01/72652 Oct., 2001 WO. Other References Masanari Takahashi et al., "Pt-TIO.sub.2 Thin Films on Glass Substrates as Efficient Photocatalysts," Journal of Materials Science, 24 (1988) pp. 243-246. S. Fukayama et al., Highly Transparent and Photoactive TIO.sub.2 Thin Film Coated on Glass Substrate, Abstract No. 735, pp. 1102-1103, 187.sup.th Electrochemical Society Meeting, Reno, NV, May 21-26, 1995, and attached fascimile transmission from The British Library indicating that the date of availability for public use was Mar. 29, 1995. D.R. Uhlmann, "Glass: Science and Technology," vol. 2, Processing I, pp. 253-283, 1984. GlassFAQs, GlassFACTS.com, pg. 1-5. JP 88-158890, Abstract Only, Derwent JP 860243762, Glass Article Difficult Soil Coating Thin Titanium Di Oxide Coating Contain Platinum@ Rhodium Palladium@. Chemical Abstracts,, 116: 89612a, Sokolov, et al., "Selective Activation of Smooth Surfaces of Glass and Ceramic Articles Before Chemical Metalization," Abstract Only. International Search Report for PCT/FR96/01421. International Preliminary Examination Report for PCT/FR96/01421. H.H. Dunken, Glass Surfaces, Treatise on Materials Science and Technology, vol. 22, pp. 1-75, 1982. Toshiya Watanabe et al., Photocatalytic Activity of TIO2 Thin Film under Room Light, in Photocatalytic Purification and Treatment of Water and Air, pp. 747-751, 1993, Ollis et al., Eds. The Japan Times, May 21, 2002, Tuesday, Race for Technology, pp. 2-5, from LexisNexis. P. Chartier, Verre, Glass Institute, vol. 3, No. 3-Jun. 1997, pp. 5-12, French document with English Translation. Pt-TiO.sub.2 Thin Films on Glass Substrates as Efficient Photocatalysts, Journal of Materials Science, 24 (1989), pp. 243-246, Document DI cited in the Opposition under Tab A. EP A 581-216, Okada et al., Feb. 2, 1994, Document D2 cited in the Opposition under Tab A; Document D17 cited in the Opposition under Tab C. Preparation of TiO.sub.2 Fine Particles Supported on Silica Gel as Photocatalyst, Yasumori et al., Journal of the Ceramic Society of Japan, 102 (1994), Document D5 cited in the Opposition under Tab A. Photocatalytic Properties of TiO.sub.2, Wold, Chem. Mater., 1993, 5, pp. 280-283, Document D6 cited in the Opposition under Tab A. Kristallstruktur und Optische Eigenshaften von Dunnen Organogenen Titanoxyd-Schichten Auf Glasunterlagen [Crystal Structure and Optical Properties of Thin Organogenic Titanium Oxide Layers on Glass Substrates], Bach et al., Thin Solid Films I (1967/68), pp. 255-276, and English tranlation, Document D7 cited in the Opposition under Tab A. US 5,165,972, Porter, Nov. 24, 1992, Document D8 cited in the Opposition under Tab A. US 4,485,146, Mizuhashi et al., Nov. 27, 1984, Document D9 cited in the Opposition under Tab A. EP 816 466, Hayakawa et al., Jan. 7, 1998, Document D11 cited in the Opposition under Tab A; Document D4 cited in the Opposition under Tab C; Document D2 cited in the Opposition under Tab D. The Effect of Substrate Temperature on the Properties of Sputtered Titanium Oxide Films, Meng et al., Applied Surface Science 65/66 (1993) pp. 235-239, Document D12 cited in the Opposition under Tab A. Sol-Gel-Derived TiO.sub.2 Film Semiconductor Electrode for Photocleavage of Water, Yoko et al., J. Electrochem Soc., vol. 138(8), Aug. 1991, pp. 2279-2284, Document D13 cited in the Opposition under Tab A. Dip-Coating of TiO.sub.2 Films Using a Sol Derived From Ti(O-iPr).sub.4 -diethamolamine-H.sub.2 O-i-PrOH System, Takahashi et al., Journal of Materials Science 23 (1988), pp. 2259-2266, Document D14 cited in the Opposition under Tab A. A Structural Investigation of Titanium Dioxide Photocatalysts, Bickley, et al., Journal of Solid State Chemistry 92, 1991, pp. 178-190, Document D15 cited in the Opposition under Tab A. Highly Transparent and Photoactive TiO2 Thin Film Coated on Glass Substrate, Fukayama et al., Abstract 735, 187.sup.th Electrochemical Society Meeting, Mar. 29, 1995, Document D4 cited in the Opposition under Tab A; Document D1 cited in the Opposition under Tab B; Document D5a cited in the Opposition under Tab C. EP 684,075, Wantanabe et al., Jun. 15, 1995 and the front page of WO 95/15816, Watanabe et al., Jun. 15, 1995, which is an equivalent of EP 684,075 and contains an English abstract, Document D10 cited in the Opposition under Tab A; Document D2 cited in the Opposition under Tab B; Document D4 cited in the Opposition under Tab D. EP 650 938 A1, Boire et al., May 3, 1995, Document D3 cited in the Opposition under Tab B. Oxide Layers Deposited from Organic Solutions, H. Schroeder in Physics of Thin Films: Advances in Research and Development, pp. 105-112, vol. 5, 1969 Academic Press, Document D4 cited in the Opposition under Tab B; Document D5 cited in the Opposition under Tab D. EP 737 531A1, Fujishima et al., Oct. 16, 1996, Document D3 cited in the Opposition under Tab A; Document D5 cited in the Opposition under Tab B; Document D16 cited in the Opposition under Tab C. JP 267476/94, Fujishima et al., publication date not listed, and Derwent WPIndex abstract; corresponds to EP 737 513A, (submitted herewith under Tab 17); WO 96/13327 (of record; published on May 9, 1996); and U.S. 6,387,844 (of record), Document D5a cited in the Opposition under Tab B; Document D3 cited in the Opposition under Tab D. WO 97/07069, Heller, Feb. 27, 1997, Document D6 cited in the Opposition under Tab B; Opposition under Tab B; Document D1 cited in the Opposition under Tab D. JP 63-100042, Kume et al., May 2, 1988, Document D7 cited in the Opposition under Tab B; Document D8 cited in the Opposition under Tab C. EP 0 071 865A2, Mizuhashi et al., Feb. 16, 1983, Document D8 cited in the Opposition under Tab B. GB 2,031,756A, Gordon, Apr. 30, 1980, Document D9 cited in the Opposition under Tab B. EP 0 348 185 B, Jenkins et al., Dec. 27, 1989, Document D10 cited in the Opposition under Tab B. JPA 63-5301 Matsushita Electric Works (assignee), Jan. 11, 1988, and Derwent WPIndex abstract, Document D2 cited in the Opposition under Tab C. JPA 63-5304, Matsushita Electric Works (assignee), Jan. 11, 1988, and Derwent WPIndex abstract, Document D7 cited in the Opposition under Tab C. JP 91042/1986, Yokoishi, May 9, 1986, Document D9 cited in the Opposition under Tab C. JPA 7-222928, Chikuni et al., Aug. 22, 1995, and Derwent WPIndex abstrac; equivalent of WO 95/15816 (submitted herewith)) and U.S. 5,853,866 (of record), 6,210,779 (submitted hereiwth), 6,294,246 (submitted herewith), and 6,294,247 (submitted herewith), Document D11 cited in the Opposition under Tab C. JPA 1-218635, Hitachi LTD (assignee), Feb. 29, 1988, and Derwent WPIndex abstract, Document D12 cited in the Opposition under Tab C. JPA 7-111104, Fujishima et al., Apr. 25, 1995, and Derwent WPIndex abstract, Document D14 cited in the Opposition under Tab C. Preparation of Transparent TiO.sub.2 Thin Film Photocatalyst and Its Photocatalytic Activity, Negishi et al., Chemistry Letters 1995, No. 9, pp. 841-842, Document D3a cited in the Oppositon under Tab D. EP 633 064, Murasawa et al., Jan. 11, 1995, Document D3 cited in the Opposition under Tab C. EP 590 477, Ogawa et al., Apr. 6, 1994, Document D10 cited in the Opposition under Tab C; Document D6 cited in the Opposition under Tab D. EP 675 086, Okada et al., Oct. 4, 1995, Document D7 cited in the Opposition under Tab D. JP-A-5 253 544, Toto LTD (assignee), Oct. 5, 1995, and Derwent WPIndex abstract, Document D1 cited in the Oppositon under Tab C; Document D8 cited in the Opposition under Tab D. JP-A-6-65012, Agency of Ind. Sci. & Technology (assignee), Mar. 8, 1994, and Derwent WPIndex abstract, Document D6 cited in the Opposition under Tab C; Document D9 cited in the Opposition under Tab D. US 4,898,789, Finley, Feb. 6, 1990, Document D10 cited in the Opposition under Tab D. EP 636 702, Shimizu et al., Feb. 2, 1995, Document D11 cited in the Opposition under Tab D. US 5,348,805, Zagdoun et al., Sep. 20, 1994, Document D12 cited in the Opposition under Tab D. Electrical and Electrochemical Properties of TiO.sub.2 films Grown by Organometallic Chemical Vapour Deposition, Takahashi et al., J. Chem. Soc., Farady Trans 1, 1982, 78, pp. 2563-2571, Document D13, cited in the Opposition under Tab D. US 4,997,576, Heller et al., Mar. 5, 1991, Document D14 cited in the Opposition under Tab D. US 5,342,676, Zagdoun, Aug. 30, 1994, Document D15 cited in the Opposition under Tab D. EP 489 621, Zagdoun et al., Jun. 6, 1992, Document D13 cited in the Opposition under Tab C. Apr. 30, 2000 Letter from Mrs. Colette Ward, Patent Litigation Enquiries, British Library, Document CW1 cited in the Opposition under Tab B. WO 95/15816, Watanabe et al., Jun. 15, 1995, Equivalent to JPA 7-222928 submitted herewith under Tab 27. US 6,210,779, Watanabe et al., Apr. 3, 2001, Equivalent to JPA 7-222928 submitted herewith under Tab 27. US 6,294,246, Wantanabe et al., Sep. 25, 2001, Equivalent to JPA 7-222928 submitted herewith under Tab 27. US 6,294,247, Wantanabe et al., Sep. 25, 2001, Equivalent to JPA 7-222928 submitted herewith under Tab 27. Primary Examiner: McNeil; Jennifer Attorney, Agent or Firm: Oblon, Spivak, McClelland, Maier & Neustadt, P.C. Parent Case Text This application is a Continuation of application Ser. No. 09/923,353, filed on Aug. 08, 2001, pending, which is a continuation of application Ser. No. 09/615,910, filed Jul. 13, 2000, now U.S. Pat. No. 6,326,079, which is a continuation of application Ser. No. 09/029,855, filed on May 28, 1998, now U.S. Pat. No. 6,103,363, which was originally filed as PCT/FR96/01421 on Sep. 13, 1996. Claims What is claimed is: 1. A coated substrate which is a glass, ceramic or vitro-ceramic substrate provided on at least a portion of one of its faces with a coating having photocatalytic properties, and comprising titanium oxide at least partially crystallized in the anatase form, and wherein the coating has a refractive index of from about 2.30 to about 2.35. 2. The coated substrate of claim 1, which is a glass substrate. 3. The coated substrate of claim 1, which is a ceramic substrate. 4. The coated substrate of claim 1, which is a vitro-ceramic substrate. 5. The coated substrate of claim 1, wherein the titanium oxide is in the form of crystallites having an average size of between 0.5 and 100 nm. 6. The coated substrate of claim 1, wherein the titanium oxide is in the form of crystallites having an average size of between 1 and 50 nm. 7. The coated substrate of claim 1, wherein the titanium oxide is in the form of crystallites having an average size of between 10 and 40 nm. 8. The coated substrate of claim 1, wherein the titanium oxide is in the form of crystallites having an average size of between 20 and 30 nm. 9. The coated substrate of claim 1, wherein coating is deposited by chemical vapor deposition followed by annealing. 10. The coated substrate of claim 1, wherein the coating has a thickness between 5 nm and 1 micron. 11. The coated substrate of claim 1, wherein the coating has a thickness between 5 nm and 100 nm. 12. The coated substrate of claim 1, wherein the coating has a thickness between 10 and 80 nm. 13. The coated substrate of claim 1, wherein the coating has a thickness between 20 and 50 nm. 14. A process for a producing a coated substrate as claimed in claim 1, comprising: chemical vapor depositing at least one titanium precursor on at least part of at least one face of a glass-, ceramic-, or vitroceramic-based substrate, to produce a photocatalytic coating containing at least partially crystalline titanium oxide, wherein the crystalline titanium oxide is in the form of crystallites with an average size of between 0.5 and 100 nm, and the thickness of the coating is between 5 nm and 1 micron. 15. The process of claim 14, wherein the substrate is glass. 16. The process of claim 14, wherein the substrate is a float-glass. 17. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide has a degree of porosity of 65 to 99%. 18. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide has a degree of porosity of 70 to 99%. 19. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide has a degree of porosity of 70 to 90%. 20. The process of claim 14, wherein the crystalline titanium oxide is in the form of crystallites with an average size of between 0.5 and 60 nm. 21. The process of claim 14, wherein the crystalline titanium oxide is in the form of crystallites with an average size of between 1 and 50 nm. 22. The process of claim 14, wherein the crystalline titanium oxide is in the form of crystallites with an average size of between 10 and 40 nm. 23. The process of claim 14, wherein the crystalline titanium oxide is in the form of crystallites with an average size of between 20 and 30 nm. 24. The process of claim 14, wherein the thickness of the coating containing the at least partially crystalline titanium oxide is between 5 nm and 100 nm. 25. The process of claim 14, wherein the thickness of the coating containing the at least partially crystalline titanium oxide is 10 nm to 80 nm. 26. The process of claim 14, wherein the thickness of the coating containing the at least partially crystalline titanium oxide is between 5 nm and 20 nm. 27. The process of claim 14, wherein the crystalline titanium oxide is in the form of crystallites and the thickness of the coating containing the at least partially crystalline titanium oxide is at least two times greater than the mean diameter of the crystallites. 28. The process of claim 14, wherein the crystalline titanium oxide is in the anatase form, in the rutile form, or in the form of a mixture of anatase and rutile. 29. The process of claim 14, wherein the titanium oxide is crystalline with a degree of crystallization of at least 25%. 30. The process of claim 14, wherein the titanium oxide is crystalline with a degree of crystallization of between 30 and 80%. 31. The process of claim 14, wherein the surface of the coating containing the at least partially crystalline titanium oxide has a contact angle with water of less than 5 after exposure to light radiation. 32. The process of claim 14, wherein the surface of the coating containing the at least partially crystalline titanium oxide has a contact angle with water of less than 1 after exposure to light radiation. 33. The process of claim 14, wherein the RMS roughness of the coating containing the at least partially crystalline titanium oxide is between 2 and 20 nm. 34. The process of claim 14, wherein the RMS roughness of the coating containing the at least partially crystalline titanium oxide is between 5 and 20 nm. 35. The process of claim 14, wherein the chemical vapor deposition is conducted at a temperature of 400 to 600.degree. C. 36. The process of claim 14, wherein the chemical vapor deposition is conducted at a temperature of 400 to 600.degree. C. 37. The process of claim 14, wherein the titanium precursor is a titanium halide. 38. The process of claim 14, wherein the titanium precursor is an organometallic titanium compound. 39. The process of claim 14, wherein the titanium precursor is a titanium alcoholate. 40. The process of claim 14, wherein the titanium precursor is TiCl4. 41. The process of claim 14, wherein the titanium precursor is selected from the group consisting of Ti(OiPr)4, titanium diisopropoxide diacetylacetonate, titanium tetraoctyleneglycolate, and titanium acetylacetonate. 42. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide also contains at least one additive capable of accentuating the photocatalytic activity of the titanium oxide. 43. The process of claim 14, further comprising incorporating metal particles or particles based on such a metal into the coating, wherein the metal is selected from the group consisting of cadmium, tin, tungsten, zinc, cerium, and zirconium. 44. The process of claim 14, further comprising inserting into the crystal lattice of the titanium oxide at least one the metal is selected from the group consisting of niobium, tantalum, iron, bismuth, cobalt, nickel, copper, ruthenium, cerium, and molybdenum. 45. The process of claim 14, further comprising covering at least part of the coating containing the at least partially crystalline titanium oxide with a layer of metal oxides or salts, wherein the metal is selected from the group consisting of iron, copper, ruthenium, cerium, molybdenum, vanadium, and bismuth. 46. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide also contains an amorphous or partially crystalline oxide or mixture of oxides of the silicon oxide, tin oxide, zirconium oxide, or aluminum oxide type. 47. The process of claim 14, further comprising coating at least a portion of the coating containing the at least partially crystalline titanium oxide with a noble metal. 48. The process of claim 47, wherein the noble metal is selected from the group consisting of platinum, rhodium, silver, and palladium. 49. The process of claim 14, wherein the substrate is coated with at least one thin layer, wherein the thin layer has an anti-static function, a thermal function or an optical function, or forms a barrier to the migration of alkali metals; and the coating containing the at least partially crystalline titanium oxide is coated on the thin layer. 50. The process of claim 49, wherein the thin layer has an anti-static function. 51. The process of claim 50, wherein the thin layer is based on a conductive material of the metal type or of the doped metal oxide type. 52. The process of claim 51, wherein the thin layer is based on ITO, SnO2:F, ZnO:F, ZnO:Al, ZnO:Sn or a metal oxide which is stoichiometrically deficient in oxygen. 53. The process of claim 52, wherein the metal oxide which is stoichiometrically deficient in oxygen is SnO2-x or ZnO2-x, wherein x<2. 54. The process of claim 49, wherein the thin layer has a thermal function. 55. The process of claim 54, wherein the thin layer is based on a conductive material of the metal type or of the doped metal oxide type. 56. The process of claim 55, wherein the thin layer is based on ITO, SnO2:F, ZnO:F, ZnO:Al, ZnO:Sn or a metal oxide which is stoichiometrically deficient in oxygen. 57. The process of claim 56, wherein the metal oxide which is stoichiometrically deficient in oxygen is SnO2-x or ZnO2-x, wherein x<2. 58. The process of claim 49, wherein the thin layer has an optical function. 59. The process of claim 58, wherein the thin layer is based on a conductive material of the metal type or of the doped metal oxide type. 60. The process of claim 59, wherein the thin layer is based on ITO, SnO2:F, ZnO:F, ZnO:Al, ZnO:Sn or a metal oxide which is stoichiometrically deficient in oxygen. 61. The process of claim 60, wherein the metal oxide which is stoichiometrically deficient in oxygen is SnO2-x or ZnO2-x, wherein x<2. 62. The process of claim 58, wherein the thin layer is based on an oxide or on a mixture of oxides with a refractive index which is intermediate between that of the coating containing the at least partially crystalline titanium oxide and that of the substrate. 63. The process of claim 62, wherein the thin layer is composed of A1203, SnO2, 1n203, silicon oxycarbide, or silicon oxynitride. 64. The process of claim 49, wherein the thin layer forms a barrier to the migration of alkali metals. 65. The process of claim 64, wherein the thin layer is based on silicon oxide, silicon nitride, silicon oxynitride, silicon oxycarbide, A1203:F, or aluminum nitride. 66. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide is the final layer of a stack of anti-glare layers. 67. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide is deposited in at least two successive stages. 68. The process of claim 14, wherein the coating containing the at least partially crystalline titanium oxide is subjected, after deposition, to at least one heat treatment of the annealing type. Description The invention relates to glass-, ceramic- or vitroceramic-based substrates, more particularly made of glass, in particular transparent substrates, which are furnished with coatings with photocatalytic properties, for the purpose of manufacturing glazing for various applications, such as utilitarian glazing or glazing for vehicles or for buildings. There is an increasing search to functionalize glazing by depositing at the surface thereof thin layers intended to confer thereon a specific property according to the targeted application. Thus, there exist layers with an optical function, such as so-called anti-glare layers composed of a stack of layers alternatively with high or low refractive indices. For an anti-static function or a heating function of the anti-icer type, it is also possible to provide eleclxically conducting thin layers, for example based on metal or doped metal oxide. For an anti-solar or low-emissivity thermal function for example, thin layers made of metal of the silver type or based on metal oxide or nitride may be used. To obtain a "rain-repellent" effect, it is possible to provide layers with a hydrophobic nature, for example based on fluorinated organosilane and the like. However, there still exists a need for a substrate, particularly a glazing, which could be described as "dirt-repellent", that is to say targeted at the permanence over time of the appearance and surface properties, and which makes it possible in particular to render cleaning less frequent and/or to improve the visibility, by succeeding in removing, as they are formed, the dirty marks which are gradually deposited at the surface of a substrate, in particular dirty marks of organic origin, such as finger marks or volatile organic products present in the atmosphere, or even dirty marks of condensation type. In point of fact, it is known that there exist certain semiconductive materials based on metal oxides which are capable, under the effect of radiation of appropriate wavelength, of initiating radical reactions which cause the oxidation of organic products; they are generally referred to as "photocatalytic" or alternatively "photoreactive" materials. The aim of the invention is then to develop photocatalytic coatings on a substrate which exhibit a marked "dirt-repellent" effect with respect to the substrate and which can be manufactured industrially. The object of the invention is a glass-, ceramic- or vitroceramic-based substrate, in particular made of glass and transparent, provided on at least part of at least one of its faces with a coating with a photocatalytic property containing at least partially crystalline titanium oxide. The titanium oxide is preferably crystallized "in situ" during the formation of the coating on the substrate. Titanium oxide is in fact one of the semi-conductors which, under the effect of light in the visible or ultraviolet range, degrade organic products which are deposited at their surface. The choice of titanium oxide to manufacture a glazing with a "dirt-repellent" effect is thus particularly indicated, all the more so since this oxide exhibits good mechanical strength and good chemical resistance: for long-term effectiveness, it is obviously important for the coating to retain its integrity, even if it is directly exposed to numerous attacks, in particular during the fitting of the glazing on a building site (building) or on a production line (vehicle) which involves repeated handlings by mechanical or pneumatic prehension means, and also once the glazing is in place, with risks of abrasion (windscreen wipers, abrasive rag) and of contact with aggressive chemicals (atmospheric pollutants of SO.sub.2 type, cleaning product, and the like). The choice has fallen, in addition, on a titanium oxide which is at least partially crystalline because it has been shown that it had a much better performance in terms of photocatalytic property than amorphous titanium oxide. It is preferably crystallized in the anatase form, in the rutile form or in the form of a mixture of anatase and rutile, with a degree of crystallization of at least 25%, in particular of approximately 30 to 80%, in particular close to the surface (the property being rather a surface property). (Degree of crystallization is understood to mean the amount by weight of crystalline TiO.sub.2 with respect to the total amount by weight of TiO.sub.2 in the coating). It has also been possible to observe, in particular in the case of crystallization in anatase form, that the orientation of the TiO.sub.2 crystals growing on the substrate had an effect on the photocatalytic behaviour of the oxide: there exists a favoured orientation (1, 1, 0) which markedly promotes photocatalysis. The coating is advantageously manufactured so that the crystalline titanium oxide which it contains is in the form of "crystallites", at least close to the surface, that is to say of monocrystals, having an average size of between 0.5 and 100 nm, preferably 1 to 50 nm, in particular 10 to 40 nm, more particularly between 20 and 30 nm. It is in fact in this size range that titanium oxide appears to have an optimum photo-catalytic effect, probably because the crystallites of this size develop a high active surface area. As will be seen in more detail subsequently, it is possible to obtain the coating based on titanium oxide in many of ways: by decomposition of titanium precursors (pyrolysis techniques: liquid pyrolysis, powder pyrolysis, pyrolysis in the vapour phase, known as CVD (Chemical Vapour Deposition), or techniques associated with the sol-gel: dipping, cell coating, and the like), by a vacuum technique (reactive or non-reactive cathodic sputtering). The coating can also contain, in addition to the crystalline titanium oxide, at least one other type of inorganic material, in particular in the form of an amorphous or partially crystalline oxide, for example a silicon oxide (or mixture of oxides), titanium oxide, tin oxide, zirconium oxide or aluminium oxide. This inorganic material can also participate in the photo-catalytic effect of the crystalline titanium oxide, by itself exhibiting to a certain extent a photocatalytic effect, even a weak effect compared with that of crystalline TiO.sub.2, which is the case with tin oxide or amorphous titanium oxide. A layer of "mixed" oxide thus combining at least partially crystalline titanium oxide with at least one other oxide can be advantageous from an optical viewpoint, very particularly if the other oxide or oxides are chosen with a lower index than that of TiO.sub.2 : by lowering the "overall" refractive index of the coating, it is possible to vary the light reflection of the substrate provided with the coating, in particular to lower this reflection. This is the case if, for example, a layer made of TiO.sub.2 /Al.sub.2 O.sub.3, a method for the preparation of which is described in Patent EP-0,465,309, or made of TiO.sub.2 /SiO.sub.2 is chosen. It is necessary, of course, for the coating to contain however a TiO.sub.2 content which is sufficient to maintain a significant photocatalytic activity. It is thus considered that it is preferable for the coating to contain at least 40% by weight, in particular at least 50% by weight, of TiO.sub.2 with respect to the total weight of oxide(s) in the coating. It is also possible to choose to superimpose, with the coating according to the invention, a grafted oleophobic and/or hydrophobic layer which is stable or resistant to photocatalysis, for example based on the fluorinated organosilane described in U.S. Pat. No. 5,368,892 and U.S. Pat. No. 5,389,427 and on the perfluoroalkylsilane described in Patent Application FR 94/08734 of Jul. 13, 1994, published under the number FR-2,722,493 and corresponding to European Patent EP-0,692,463, in particular of formula: CF.sub.3 --(CF.sub.2).sub.n --(CH.sub.2).sub.m --SiX.sub.3 in which n is from 0 to 12, m is from 2 to 5 and X is a hydrolysable group. To amplify the photocatalytlc effect of the titanium oxide of the coating according to the invention, it is possible first of all to increase the absorption band of the coating, by incorporating other particles in the coating, in particular metal particles or particles based on cadmium, tin, tungsten, zinc, cerium or zirconium. It is also possible to increase the number of charge carriers by doping the crystal lattice of the titanium oxide by inserting therein at least one of the following metal elements: niobium, tantalum, iron, bismuth, cobalt, nickel, copper, ruthenium, cerium or molybdenum. This doping can also be carried out by surface doping only of the titanium oxide or of the combined coating, surface doping carried out by covering at least part of the coating with a layer of metal oxides or salts, the metal being chosen from iron, copper, ruthenium, cerium, molybdenum, vanadium and bismuth. Finally, the photocatalytic phenomenon can be accentuated by increasing the yield and/or the kinetics of the photocatalytic reactions, by covering the titanium oxide, or at least part of the coating which incorporates it, with a noble metal in the form of a thin layer of the platinum, rhodium, silver or palladium type. Such a catalyst, for example deposited by a vacuum technique, in fact makes it possible-to increase the number and/or the lifetime of the radical entities created by the titanium oxide and thus to promote the chain reactions leading to the degradation of organic products. In an entirely surprising way, the coating exhibits in fact not one property but two, as soon as it is exposed to appropriate radiation, as in the visible and/or ultraviolet field, such as sunlight: by the presence of photocatalytic titanium oxide, as already seen, it promotes the gradual disappearance, as they are accumulated, of dirty marks of organic origin, their degradation being caused by a radical oxidation process. Inorganic dirty marks are not, themselves, degraded by this process: they therefore remain on the surface and, except for a degree of crystallization, they are in part easily removed since they no longer have any reason to adhere to the surface, the binding organic agents being degraded by photocatalysis. However, the coating of the invention, which is permanently self-cleaning, also preferably exhibits an external surface with a pronounced hydrophilic and/or oleophilic nature which results in three very advantageous effects: hydrophilic nature makes possible complete wetting of the water which can be deposited on the coating. When a water condensation phenomenon takes place, instead of a deposit of water droplets in the form of condensation which hampers visibility, there is in fact a continuous thin film of water which is formed on the surface of the coating and which is entirely transparent. This "anti-condensation" effect is in particular demonstrated by the measurement of a contact angle with water of less than 5.degree. after exposure to light, and after running of water, in particular of rain,; over a surface which has not been treated with a photocatalytic layer, many drops of rainwater remain stuck to the surface and leave, once evaporated, unattractive and troublesome marks, mainly of inorganic origin. Indeed, a surface exposed to the surrounding air is rapidly covered by a layer of dirty marks which limits the wetting thereof by water. These dirty marks are in addition to the other dirty marks, in particular inorganic marks (crystallizations and the like), contributed by the atmosphere in which the glazing bathes. In the case of a photoreactive surface, these inorganic dirty marks are not directly degraded by photocatalysis. In fact, they are in very large part removed by virtue of the hydrophilic nature induced by the photo-catalytic activity. This hydrophilic nature indeed causes complete spreading of the drops of rain. Evaporation marks are therefore no longer present. Moreover, the other inorganic dirty marks present on the surface are washed, or redissolved in the case of crystallization, by the water film and are thus in large part removed. An "inorganic dirt-repellent" effect is obtained, induced in particular by rain, in conjunction with a hydrophilic nature, the coating can also exhibit an oleophilic nature which makes possible the "wetting" of the organic dirty marks which, as with water, then tend to be deposited on the coating in the form of a continuous film which is less visible than highly localized "stains". An "organic dirt-repellent" effect is thus obtained which operates in two ways: as soon as it is deposited on the coating, the dirty mark is already not very visible. Subsequently, it gradually disappears by radical degradation initiated by photocatalysis. The coating can be chosen with a more or less smooth surface. A degree of roughness can indeed be advantageous: it makes it possible to develop a greater active photocatalytic surface area and thus induces a greater photocatalytic activity, it has a direct effect on the wetting. The roughness in fact enhances the wetting properties. A smooth hydophilic surface will be even more hydrophilic once rendered rough. "Roughness" is understood to mean, in this instance, both the surface roughness and the roughness induced by a porosity of the layer in at least a portion of its thickness. The above effects will be all the more marked when the coating is porous and rough, resulting in a superhydrophilic effect for rough photoreactive surfaces. However, when exaggerated, the roughness can be penalizing by promoting incrustation or accumulation of dirty marks and/or by bringing about the appearance of an optically unacceptable level of fuzziness. It has thus proved to be advantageous to adapt the method for deposition of TiO.sub.2 -based coatings so that they exhibit a roughness of approximately 2 to 20 nm, preferably of 5 to 15 nm, this roughness being evaluated by atomic force microscopy, by measurement of the value of the root mean square or RMS over a surface area of 1 square micrometre. With such roughnesses, the coatings exhibit a hydrophilic nature which is reflected by a contact angle with water which can be less than 1.degree.. It has also been found that it is advantageous to promote a degree of porosity in the thickness of the coating. Thus, if the coating consists only of TiO.sub.2, it preferably exhibits a porosity of the order of 65 to 99%, in particular of 70 to 90%, the porosity being defined in this instance indirectly by the percentage of the theoretical relative density of TiO.sub.2, which is approximately 3.8. One means for promoting such a porosity comprises, for example, the deposition of the coating by a technique of the sol-gel type involving the decomposition of materials of organometallic type: an organic polymer of polyethylene glycol PEG type can then be introduced into the solution, in addition to the organometallic precursor(s): on curing the layer by heating, the PEG is burnt off, which brings about or accentuates a degree of porosity in the thickness of the layer. The thickness of the coating according to the invention is variable; it is preferably between 5 nm and 1 micron, in particular between 5 and 100 nm, in particular between 10 and 80 nm, or between 20 and 50 nm. In fact, the choice of the thickness can depend on various parameters, in particular on the targeted application of the substrate of the glazing type or alternatively on the size of the TiO.sub.2 crystallites in the coating or on the presence of a high proportion of alkali metals in the substrate. It is possible to arrange, between the substrate and the coating according to the invention, one or a number of other thin layers with a different or complementary function to that of the coating. It can concern, in particular, layers with an anti-static, thermal or optical function or promoting the crystal-line growth of TiO.sub.2 in the anatase or rutile form or of layers forming a barrier to the migration of certain elements originating from the substrate, in particular forming a barrier to alkali metals and very particularly to sodium ions when the substrate is made of glass. It is also possible to envisage a stack of alternating "anti-glare" layers of thin layers with high and low indices, the coating according to the invention constituting the final layer of the stack. In this case, it is preferable for the coating to have a relatively low refractive index, which is the case when it is composed of a mixed oxide of titanium and of silicon. The layer with an anti-static and/or thermal function (heating by providing it with power leads, low-emissive, anti-solar, and the like) can in particular be chosen based on a conductive material of the metal type, such as silver, or of the doped metal oxide type, such as indium oxide doped with tin ITO, tin oxide doped with a halogen of the fluorine type SnO.sub.2 :F or with antimony SnO.sub.2 :Sb or zinc oxide doped with indium ZnO:In, with fluorine ZnO:F, with aluminium ZnO:Al or with tin ZnO:Sn. It can also concern metal oxides which are stoichiometrically deficient in oxygen, such as SnO.sub.2-x or ZnO.sub.2-x with x<2. The layer with an anti-static function preferably has a surface resistance value of 20 to 1000 ohms.square. Provision can be made for furnishing it with power leads in order to polarize it (feeding voltages for example of between 5 and 100 V). This controlled polarization makes it possible in particular to control the deposition of dust with a size of the order of a millimeter capable of being deposited on the coating, in particular dry dust which adheres only by an electrostatic effect: by suddenly reversing the polarization of the layer, this dust is "ejected". The thin layer with an optical function can be chosen in order to decrease the light reflection arid/or to render more neutral the colour in reflection of the substrate. In this case, it preferably exhibits a refractive index intermediate between that of the coating and that of the substrate and an appropriate optical thickness and can be composed of an oxide or of a mixture of oxides of the aluminium oxide Al.sub.2 O.sub.3, tin oxide SnO.sub.2, indium oxide In.sub.2 O.sub.3 or silicon oxycarbide or oxynitride type. In order to obtain maximum attenuation of the colour in reflection, it is preferable for this thin layer to exhibit a refractive index close to the square root of the product of the squares of the refractive indices of the two materials which frame it, that is to say the substrate and the coating according to the invention. In the same way, it is advantageous to choose its optical thickness (that is to say the product of its geometric thickness and of its refractive index) similar to lambda/4, lambda being approximately the average wavelength in the visible, in particular from approximately 500 to 550 nm. The thin layer with a barrier function with respect to alkali metals can be in particular chosen based on silicon oxide, nitride, oxynitride or oxycarbide, made of aluminium oxide containing fluorine Al.sub.2 O.sub.3 :F or alternatively made of aluminium nitride. In fact, it has proved to be useful when the substrate is made of glass, because the migration of sodium ions into the coating according to the invention can, under certain conditions, detrimentally affect the photocatalytic properties thereof. The nature of the substrate or of the sublayer furthermore has an additional advantage: it can promote the crystallization of the photocatalytic layer which is deposited, in particular in the case of CVD deposition. Thus, during deposition of TiO.sub.2 by CVD, a crystalline SnO.sub.2 :F sublayer promotes the growth of TiO.sub.2 mostly in the rutile form, in particular for deposition temperatures of the order of 400.degree. to 500.degree. C., whereas the surface of a soda-lime glass or of a silicon oxycarbide sublayer rather induces an anatase growth, in particular for deposition temperatures of the order of 400.degree. to 600.degree. C. All these optional thin layers can, in a known way, be deposited by vacuum techniques of the cathodic sputtering type or by other techniques of the thermal decomposition type, such as solid, liquid or gas phase pyrolyses. Each of the abovementioned layers can combine a number of functions but it is also possible to superimpose them. Another subject of the invention is "dirt-repellent" (organic and/or inorganic dirty marks) and/or "anti-condensation" glazing, whether it is monolithic or insulating multiple units of the double glazing or laminated type, which incorporates the coated substrates described above. The invention is thus targeted at the manufacture of glass, ceramic or vitroceramic products and very particularly at the manufacture of "self-cleaning" glazing. The latter can advantageously be building glazing, such as double glazing (it is then possible to arrange the coating "external side" and/or "internal side", that is to say on face 1 and/or on face 4). This proves to be very particularly advantageous for glazing which is not very accessible to cleaning and/or which needs to be cleaned very frequently, such as roofing glazing, airport glazing, and the like. It can also relate to vehicle windows where maintenance of visibility is an essential safety criterion. This coating can thus be deposited on car windscreens, side windows or rear windows, in particular on the face of the windows turned towards the inside of the passenger compartment. This coating can then prevent the formation of condensation and/or remove traces of dirty finger mark, nicotine or organic material type, the organic material being of the volatile plasticizing type released by the plastic lining the interior of the passenger compartment, in particular that of the dashboard (release sometimes known under the term "fogging"). Other vehicles such as planes or trains can also find it advantageous to use windows furnished with the coating of the invention. A number of other applications are possible, in particular for aquarium glass, shop windows, green houses, verandas, or glass used in interior furniture or street furniture but also mirrors, television screens, the spectacle field or any architectural material of the facing material, cladding material or roofing material type, such as tiles, and the like. The invention thus makes it possible to funcationalize these known products by conferring on them anti-ultraviolet, dirt-repellent, bactericidal, anti-glare, anti-static or antimicrobial properties and the like. Another advantageous application of the coating according to the invention consists in combining it with an electrically controlled variable absorption glazing of the following types: electrochromic glazing, liquid crystal glazing, optionally with dichroic dye, glazing containing a system of suspended particles, viologen glazing and the like. As all these glazing types are generally composed of a plurality of transparent substrates, between which are arranged the "active" elements, it is then possible advantageously to arrange the coating on the external face of at least one of these substrates. In particular in the case of an electrochromic glazing, when the latter is in the coloured state, its absorption results in a degree of surface heating which, in fact, is capable of accelerating the photocatalytic decomposition of the carbonaceous substances which are deposited on the coating according to the invention. For further details on the structure of an electrochromic glazing, reference will advantageously be made to Patent Application EP-A-0,575,207, which describes an electrochromic laminated double glazing, it being possible for the coating according to the invention preferably to be positioned on face 1. Another subject of the invention is the various processes for obtaining the coating according to the invention. It is possible to use a deposition technique of the pyrolysis type which is advantageous because it in particular makes possible the continuous deposition of the coating directly on the float-glass strip when a glass substrate is used. The pyrolysis can be carried out in the solid phase, from powder(s) of precursor(s) of the organo-metallic type. The pyrolysis can be carried out in the liquid phase, from a solution comprising an organometallic titanium precursor of the titanium chelate and/or titanium alcoholate type. Such precursors are mixed with at least one other organometallic precursor. For further details on the nature of the titanium precursor or on the deposition conditions, reference will be made, for example, to Patents FR-2,310,977 and EP-0,465,309. The pyrolysis can also be carried out in the vapour phase, which technique is also denoted under the term of CVD (Chemical Vapour Deposition), from at least one titanium precursor of the halide type, such as TiCl.sub.4, or titanium alcoholate of the Ti tetraisopropylate type, Ti(OiPr).sub.4. The crystallization of the layer can additionally be controlled by the type of sublayer, as mentioned above. It is also possible to deposit the coating by other techniques, in particular by techniques in combination with the "sol-gel". Various deposition methods are possible, such as "dipping", also known as "dip coating", or a deposition using a cell known as "cell coating". It can also concern a method of deposition by "spray coating" or by laminar coating, the latter technique being described in detail in Patent Application WO-94/01598. All these deposition methods in general use a solution comprising at least one organometallic precursor, in particular titanium of the. alcoholate type, which is thermally decomposed after coating the substrate with the solution on one of its faces or on both its faces. It can be advantageous, moreover, to deposit the coating, whatever the deposition technique envisaged, not in a single step but via at least two successive stages, which appears to promote the crystallization of titanium oxide throughout the thickness of the coating when a relatively thick coating is chosen. Likewise, it is advantageous to subject the coating with a photocatalytic property, after deposition, to a heat treatment of the annealing type. A heat treatment is essential for a technique of the sol-gel or laminar coating type in order to decompose the organometallic precursor(s) to oxide, once the substrate has been coated, and to improve the resistance to abrasion, which is not the case when a pyrolysis technique is used, where the precursor decomposes as soon as it comes into contact with the substrate. In the first case, as in the second, however, a post-deposition heat treatment, once the TiO.sub.2 has been formed, improves its degree of crystallization. The chosen treatment temperature can in addition make possible better control of the degree of crystallization and of the crystalline nature, anatase and/or rutile, of the oxide. However, in the case of a substrate made of soda-lime glass, multiple and prolonged annealings can promote attenuation of the photocatalytic activity because of an excessive migration of the alkali metals from the substrate towards the photoreactive layer. The use of a barrier layer between the substrate, if it is made of standard glass, and the coating, or the choice of a substrate made of glass with an appropriate composition, or alternatively the choice of a soda-lime glass with a surface from which alkali metals have been eliminated make it possible to remove this risk. Other advantageous details and characteristics of the invention emerge from the description below of non-limiting implementational examples, with the help of the following figures: FIG. 1: a cross-section of a glass substrate provided with the coating according to the invention, FIG. 2: a diagram of a sol-gel deposition technique, by so-called "dip coating" the coating, FIG. 3: a diagram of a so-called "cell coating" deposition technique, FIG. 4: a diagram of a so-called "spray coating" deposition technique, FIG. 5: a diagram of a deposition technique by laminar coating. As represented very diagrammatically in FIG. 1, all the following examples relate to the deposition of a so-called "dirt-repellent" coating 3, essentially based on titanium oxide, on a transparent substrate 1. The substrate 1 is made of clear soda-lime-silica glass with a thickness of 4 mm and a length and width of 50 cm. It is obvious that the invention is not limited to this specific type of glass. The glass can in addition not be flat but bent. Between the coating 3 and the substrate 1 is found a thin optional layer 2, either based on silicon oxycarbide, written as SiOC, for the purpose of constituting a barrier to the diffusion of the alkali metals and/or a layer which attenuates light reflection, or based on tin oxide doped with fluorine SnO.sub.2 :F, for the purpose of constituting an anti-static and/or low-emissive layer, even with a not very pronounced low-emissive effect, and/or a layer which attenuates the colour, in particular in reflection. EXAMPLES 1 TO 3 Examples 1 to 3 relate to a coating 3 deposited using a liquid phase pyrolysis technique. The operation can be carried out continuously, by using a suitable distribution nozzle arranged transversely and above the float-glass strip at the outlet of the float-bath chamber proper. In this instance, the operation is carried out non-continuously, by using a moveable nozzle arranged opposite the substrate 1 already cut to the dimensions shown, which substrate is first heated in an oven to a temperature of 400 to 650.degree. C. before progressing a constant speed past the nozzle spraying at an appropriate solution. EXAMPLE 1 In this example, there is no optional layer 2. The coating 3 is deposited using a solution comprising two organometallic titanium precursors, titanium diisopropoxide diacetylacetonate and titanium tetraoctyleneglycolate, dissolved in a mixture of two solvents, the latter being ethyl acetate and isopropanol. It should be noted that it is also entirely possible to use other precursors of the same type, in particular other titanium chelates of the titanium acetylacetonate, titanium (methyl acetoacetato), titanium (ethyl acetoacetato) or alternatively titanium triethanolaminato or titanium diethanolaminato type. As soon as the substrate 1 has reached the desired temperature in the oven, i.e. in particular approximately 500.degree. C., the substrate progresses past the nozzle which sprays at room temperature, using compressed air, the mixture shown. A TiO.sub.2 layer with a thickness of approximately 90 nm is then obtained, it being possible for the thickness to be controlled by the rate of progression of the substrate 1 past the nozzle and/or the temperature of the said substrate. The layer is partially crystalline in the anatase form. This layer exhibits excellent mechanical behaviour. Its resistance to abrasion tests is comparable with that obtained for the surface of the bare glass. It can be bent and dip coated. It does not exhibit bloom: the scattered light transmission of the coated substrate is less than 0.6% (measured according to the D.sub.65 illuminant at 560 nm) EXAMPLE 2 Example 1 is repeated but inserting, between the substrate 1 and coating 3, an SnO.sub.2 :F layer 2 with a thickness of 73 nm. This layer is obtained by powder pyrolysis from dibutyltin difluoride DBTF. It can also be obtained, in a known way, by pyrolysis in the liquid or vapour phase, as is for example described in Patent Application EP-A-0,648,196. In the vapour phase, it is possible in particular to use a mixture of monobutyltin trichloride and of a fluorinated precursor optionally in combination with a "mild" oxidant of the H.sub.2 O type. The index of the layer obtained is approximately 1.9. Its surface resistance is approximately 50 ohms. In the preceding Example 1, the coated substrate 1, mounted as a double glazing so that the coating is on face 1 (with another substrate 1' which is non-coated but of the same nature and dimensions as the substrate 1 via a 12 mm layer of air), exerts a colour saturation value in reflection of 26% and a colour saturation value in transmission of 6.8%. In this Example 2, the colour saturation in reflection (in the goldens) is only 3.6% and it is 1.1% in transmission. Thus, the SnO.sub.2 :F sublayer makes it possible to confer, on the substrate, anti-static properties due to its electrical conductivity and it also has a favourable effect on the colorimetry of the substrate, by making its coloration markedly more "neutral", both in transmission and in reflection, which coloration is caused by the presence of the titanium oxide coating 3 exhibiting a relatively high refractive index. It is possible to polarize it by providing it with a suitable electrical supply, in order to limit the deposition of dust with a relatively large size, of the order of a millimeter. In addition, this sublayer decreases the diffusion of alkali metals into the photocatalytic TiO.sub.2 layer. The photocatalytic activity is thus improved. EXAMPLE 3 Example 2 is repeated but this time inserting, between substrate 1 and coating 3, a layer 2 based on silicon oxycarbide with an index of approximately 1.75 and a thickness of approximately 50 nm, which layer can be obtained by CVD from a mixture of SiH.sub.4 and ethylene diluted in nitrogen, as described in Patent Application EP-A-0,518,755. This layer is particularly effective in preventing the tendency of alkali metals (Na.sup.+, K.sup.+) and of alkaline-earth metals (Ca.sup.++) originating from the substrate to diffuse towards the coating 3 and thus the photocatalytic activity is markedly improved. As it has, like SnO.sub.2 :F, a refractive index intermediate between that of the substrate (1.52) and of the coating 3 (approximately 2.30 to 2.35), it also makes it possable to reduce the intensity of the coloration of the substrate, both in reflection and in transmission, and overall to decrease the light reflection value R.sub.L of the said substrate. The following Examples 4 to 7 relate to depositions by CVD. EXAMPLES 4 TO 7 EXAMPLE 4 This example relates to the deposition by CVD of the coating 3 directly on the substrate 1 using a standard nozzle, such as that represented in the above-mentioned Patent Application EP-A-0,518,755. Use is made, as precursors, either of an organometallic compound or of a metal halide. In this instance, titanium tetraisopropylate is chosen as organometallic compound, this compound being advantageous because of its high volatility and its large working temperature range, from 300 to 650.degree. C. In this example, deposition is carried out at approximately 425.degree. C. and the TiO.sub.2 thickness is 15 nm. Tetraethoxytitanium Ti(O-Et).sub.4 may also be suitable and, as halide, mention may be made of TiCl.sub.4. EXAMPLE 5 It is carried out similarly to Example 4, except that, in this instance, the 15 nm TiO.sub.2 layer is not deposited directly on the glass but on a 50 nm SiOC sublayer deposited as in Example 3. EXAMPLE 6 It is carried out as in Example 4, except that, in this instance, the thickness of the TiO.sub.2 layer is 65 nm. EXAMPLE 7 It is carried out as in Example 5, except that, in this instance, the thickness of the TiO.sub.2 layer is 60 nm. From these Examples 4 to 7, it is found that the substrates thus coated exhibit good mechanical behaviour with respect to the abrasion tests. In particular, no delamination of the TiO.sub.2 layer is observed. EXAMPLE 8 This example uses a technique in combination with the sol-gel using a deposition method by "dipping", also known as "dip coating", the principle of which emerges from FIG. 2: it consists in immersing the substrate 1 in the liquid solution 4 containing the appropriate precursor(s) of the coating 3 and in then withdrawing the substrate 1 therefrom at a controlled rate using a motor means 5, the choice of the rate of withdrawal making it possible to adjust the thickness of solution remaining at the surface of the two faces of the substrate and, in fact, the thickness of the coatings deposited, after heat treatment of the latter in order both to evaporate the solvent and to decompose the precursor or precursors to oxide. Use is made, for depositing the coating 3, of a solution 4 comprising either titanium tetrabutoxide Ti(O-Bu).sub.4, stabilized with diethanolamine DEA in the molar proportion 1:1, in an ethanol-type solvent containing 0.2 mol of tetrabutoxide per litre of ethanol, or the mixture of precursors and of solvents described in Example 1. (Another precursor, such as titanium (diethanolaminato)dibutoxide, can also be used). The substrates 1 can contain SiOC sublayers. After withdrawal from each of the solutions 4, the substrates 1 are heated for 1 hour at 100.degree. C. and then for approximately 3 hours at 550.degree. C. with the temperature raised gradually. A coating 3 is obtained on each of the faces, which coating is in both cases made of highly crystal-line TiO.sub.2 in the anatase form. EXAMPLE 9 This example uses the technique known as "cell coating", the principle of which is recalled in FIG. 3. It relates to forming a narrow cavity, delimited by two substantially parallel faces 6,7 and two seals 8, 9, at least one of these faces 6, 7 consisting of the face of the substrate 1 to be treated. The cavity is then filled with the solution 4 of precursors) of the coating and the solution 4 is withdrawn in a controlled way, so as to form a wetting meniscus, for example using a peristaltic pump 10, leaving a film of the solution 4 on the face of the substrate 1 as this solution is withdrawn. The cavity 5 is then maintained for at least the time necessary for drying. The film is cured by heat treatment. The advantage of this technique, in comparison with "dip coating", is in particular that it is possible to treat only a single one of the two faces of the substrate 1 and not both systematically, unless a masking system is resorted to. The substrates 1 comprise thin layers 2 based on silicon oxycarbide SiOC. Example 6 uses respectively the solutions 4 described in Example 8. The same heat treatments are then carried out in order to obtain the TiO.sub.2 coating 3. The coating 3 exhibits good mechanical durability. Under an SEM (scanning electron microscope), a field effect appears in the form of "grains" of mono-crystals with a diameter of approximately 30 nm. The roughness of this coating induces wetting properties which are enhanced with respect to a non-rough coating. These same solutions 4 can also be used to deposit coatings by "spray coating", as represented in FIG. 4, where the solution 4 is sprayed in the form of a cloud against the substrate 1 statically, or by laminar coating, as represented in FIG. 5. In the latter case, the substrate 1, held by vacuum suction against a support 11 made of stainless steel and Teflon, is passed over a tank 12 containing the solution, in which solution is partially immersed a slotted cylinder 14, and the combined tank 12 and cylinder 14 are then moved over the whole length of the substrate 1, the mask 13 preventing excessive evaporation of the solvent from the solution 4. For further details regarding this latter technique, reference will advantageously be made to the abovementioned Patent Application WO-94/01598. Tests were carried out on the substrates obtained according to the above examples in order to characterize the coatings deposited and to evaluate their "anti-condensation" and "dirt-repellent" behaviour. Test 1: This is the test of the condensation aspects. It consists in observing the consequences of the photocatalysis and of the structure of the coating (level of hydroxyl groups, porosity, roughness) on the wetting. If the surface is photo-reactive, the carbonaceous microcontaminants which are deposited on the coating are continually destroyed and the surface is hydrophilic and thus anti-condensation It is also possible to carry out a quantitative evaluation by suddenly reheating the initially coated substrate, stored in the cold or simply by blowing over the substrate, by measuring if condensation appears and, in the affirmative, at what time, and by then measuring the time necessary for the disappearance of the said condensation. Test 2: It relates to the evaluation of the hydrophilicity and the oleophilicity at the surface of the coating 3, in comparison with those of the surface of a bare glass, by measurement of contact angles of a drop of water and of a drop of DOP (dioctyl phthalate) at their surfaces, after having left the substrates for one week in the surrounding atmosphere under natural light, in the dark and then having subjected them to UVA radiation for 20 minutes. Test 3: It consists in depositing, on the substrate to be evaluated, a layer of an organosilane and in irradiating it with UVA radiation so as to degrade it by photocatalysis. As the organosilane modifies the wetting properties, measurements of contact angle of the substrate with water during the irradiation indicate the state of degradation of the grafted layer. The rate of disappearance of this layer is related to the photocatalytic activity of the substrate. The grafted organosilane is a trichlorosilane: octadecyltrichlorosilane (OTS). The grafting is carried out by dipping. The test device is composed of a turntable rotating around from 1 to 6 low pressure UVA lamps. The test specimens to be evaluated are placed in the turntable, the face to be evaluated on the side of the UVA radiation. Depending on their position and the number of lamps switched on, each test specimen receives a UVA irradiation varying from 0.5 W/m.sup.2 to 50 W/m.sup.2. For Examples 1, 2, 3, 8 and 9, the irradiation power is chosen as 1.8 W/m.sup.2 and, for Examples 4 to 7, as 0.6 W/m.sup.2. The time between each measurement of the contact angle varies between 20 min and 3 h, depending on the photocatalytic activity of the test specimen under consideration. The measurements are carried out using a goniometer. Before irradiation, the glasses exhibit an angle of approximately 100.degree.. It is considered that the layer is destroyed after irradiation when the angle is less than 20.degree.. Each test specimen tested is characterized by the mean rate of disappearance of the layer, given in nanometers per hour, that is to say the thickness of the organos-lane layer deposited divided by the irradiation time which makes it possible to reach a final stationary value of less than 20.degree. (time for disappearance of the organosilane layer). All the preceding examples pass Test 1, that is to say that, when the substrates coated with the coating are blown on, they remain perfectly transparent, whereas a highly visible layer of condensation is deposited on non-coated substrates. The examples were subjected to Test 2: the coated substrates, after exposure to UVA radiation, exhibit a contact angle with water and with DOP of not more than 5.degree.. In contrast, a bare glass under the same conditions exhibits a contact angle with water of 40.degree. and a contact angle with DOP of 20.degree.. The results of the substrates coated according to the above examples in Test 3 are combined in the table below. Test 3, of wetting, at 1.8 W/m.sup.2 UVA Substrate (in nm/h) Example 1 (TiO.sub.2 on bare glass) 0.03 Example 2 (TiO.sub.2 on SnO.sub.2 :F) Example 3 (TiO.sub.2 on SiOC) Example 8 (TiO.sub.2 on 50 nm SiOC) 0.1 0.2 5 Example 9 (TiO.sub.2 on 50 nm SiOC) 5 Bare glass 0 Test 3, of wetting, at 0.6 W/m.sup.2 UVA Substrate (CVD) (in nm/h) Example 4 (TiO.sub.2 on bare glass) <0.05 nm/h Example 5 (TiO.sub.2 on SiOC) 4 Example 6 (TiO.sub.2 on bare glass) Example 7 (TiO.sub.2 on SiOC) 9 19.5 From the table, it can be seen that the presence of sublayers, in particular of SIOC, promotes the photocatalytic activity of the coating containing the TiO.sub.2, by its barrier effect to alkali metals and alkaline-earth metals which can migrate from the glass (comparison of Examples 4 and 5 or 6 and 7). It is also observed that the thickness of the coating containing the TiO.sub.2 also plays a role (comparison of Examples 1 and 3): for a TiO.sub.2 coating with a thickness greater than the mean size of the monocrystals or "crystallites", a better photocatalytic effect is obtained. Indeed, it could be observed that the TiO.sub.2 coatings obtained by CVD exhibit the most advanced crystallization, with crystallite sizes of the order of 20 to 30 nm. It can be seen that the photocatalytic activity of Example 6 (65 nm of TiO.sub.2) is markedly greater than that of Example 4 (15 nm of TiO.sub.2 only). It is therefore advantageous to provide a TiO.sub.2 coating thickness at least two times greater than the mean diameter of the crystallites which it contains. Alternatively, as in the case of Example 5, it is possible to retain a TiO.sub.2 coating with a thin thickness but then to choose to use a sublayer of an appropriate nature and with an appropriate thickness for promoting as far as possible the crystalline growth of TiO.sub.2 from the "first" layer of crystallites. It could be observed that the crystallization of the TiO.sub.2 was somewhat less advanced for the coatings deposited by a technique other than CVD. Here again, however, everything is still a matter of compromise: a less advanced crystallization and an a priori lower photocatalytic activity can be "compensated for" by the use of a deposition process which is less expensive or less complex, for example. Moreover, the use of an appropriate sublayer or the doping of the TiO.sub.2 can make it possible to improve the photocatalytic behaviour, if necessary. It is also confirmed, from the comparison of Examples 2 and 3, that the nature of the sublayer influences the crystallization form and, in fact, the photocatalytic activity of the coating. (2 United States Patent Hurst , of 4) 6,840,061 et al. January 11, 2005 Coatings on substrates Abstract A process for the production of a photocatalytically active self-cleaning coated substrate, especially a glass substrate, which comprises depositing a titanium oxide coating on the surface of the substrate by contacting it with a fluid mixture containing a source of titanium and a source of oxygen, the substrate being at a temperature of at least 600.degree. C. The coated surface has good durability, a high photocatalytic activity and a low visible light reflection. Most preferably the deposition temperature is in the range 645.degree. C. to 7200.degree. C. which provides especially good durability. The fluid mixture preferably contains titanium chloride and an ester, especially ethyl acetate. Also disclosed is a self cleaning coated substrate, especially a glass substrate, having high photocatalytic activity and low visible light reflection and a durable self-cleaning coated glass. Inventors: Hurst; Simon James (Runcorn, GB); Ammerlaan; Johannes Andreas Maria (Eindhoven, NL); McCurdy; Richard Joseph (Aurora, IL) Assignee: Libbey-Owens-Ford Co. (Toledo, OH); Pilkington PLC (Merseyside, GB) Appl. No.: 587970 Filed: June 6, 2000 Foreign Application Priority Data Jun 08, 1999[GB] 9913315 65/60.51; 65/60.5; 65/99.1; 65/99.4 Current U.S. Class: C03C 017/245 Intern'l Class: 65/60.5,60.51,60.52,60.53,99.1,99.4 Field of Search: References Cited [Referenced By] U.S. Patent Documents 4123244 Oct., 1978 Leclercq et al. 4329379 May., 1982 Terneu et al. 4351267 Sep., 1982 Kalbskopf et al. 4751149 Jun., 1988 Vijayakumar et al. 4878934 Nov., 1989 Thomas et al. 4971843 Nov., 1990 Michelotti et al. 4997576 Mar., 1991 Heller et al. 5041150 Aug., 1991 Grundy et al. 5194161 Mar., 1993 Heller et al. 5256616 Oct., 1993 Heller et al. 5308458 May., 1994 Urwin et al. 5393593 Feb., 1995 Gulotta et al. 5505989 Apr., 1996 Jenkinson. 5580364 Dec., 1996 Goodman et al. 118/305. 5853866 Dec., 1998 Watanabe et al. 5939194 Aug., 1999 Hashimoto et al. 6013372 Jan., 2000 Hayakawa et al. 6027766 Feb., 2000 Greenberg et al. 6027797 Feb., 2000 Watanabe et al. 6037289 Mar., 2000 Chopin et al. 6054227 Apr., 2000 Greenberg et al. 6090489 Jul., 2000 Hayakawa et al. 6110528 Aug., 2000 Kimura et al. Foreign Patent Documents 684075 Jun., 1995 EP. 737513 Oct., 1996 EP. 0901991 Mar., 1999 EP. 2150044 Jun., 1978 GB. 1523991 Sep., 1978 GB. 1524326 Sep., 1978 GB. 1510587 Oct., 1978 GB. 63-100042 May., 1988 JP. WO 97/07069 Feb., 1997 WO. WO 97/97069 Feb., 1997 WO. WO 97/10186 Mar., 1997 WO. WO 98/06675 Feb., 1998 WO. WO 98/41480 Sep., 1998 WO. WO 98/41480 Sep., 1998 WO. Other References Journal of Materials Science Letters 9 (1990) 316-319, Kamata et al.; "Rapid Formation of TiO.sub.2 Films By A Conventional CVD Method" no month available. Photocatalytic Activity of TiO2 Thin Film Under Room Light (in Photocatalytic Purification and Treatment of Water and Air; eds. D.F. Ollis and H, Al-Ekabi, p. 747 (1993), no month available. 427/218. Hass, Georg; Thun, Rudolf E., Ed.; Physics of Thin Films; vol. 5 Academic Press, New York, 1969, p. 237, pp. 304-306, no month available. Pierson, Hugh O.; Handbook of Chemical Vapor Deposition (CVD); Noyes Publications, Park Ridge, NJ, 1992, pp. 231-237, no month available. Takashi, Manasari et al.; "PT-TIO2 Thin Films on Glass Substrates as Efficient Photcatalysts"; J. Mat. Sci., 24 (1989) 243, no month available. Fukayama, S. etal.; "Highly Tranparent and Photoactive TIO2 Thin Film Coated on Glass Substrate"; 187th Electochemical Society Meeting, Abstract No. 735, Extened Abstracts 95-1 (Available at Least by Mar. 1995). Weinberger, B.R. et al.; "Titanium Dioxide Photocatalysts Produced by Reactive Magnetron Sputtering"; Appl. Phys. Lett. 66 (1995) 2409, no month available. Kiernan, Vincent; "A Clearer View for Car Drivers"; New Scientist, Aug. 26, 1995, p. 19. Paz, Y; Luo, A.; "Photooxideative Self-Cleaning Transparent Titanium Dioxide Films on Glass"; J. Mater.Res., 10 (Nov. 1995) 2482. Primary Examiner: Vincent; Sean Attorney, Agent or Firm: Marshall & Melhorn, LLC Claims What is claimed is: 1. A process for the production of a durable photocatalytically active coated glass which comprises depositing a photocatalytically active titanium oxide layer on the surface of a glass ribbon formed during a float glass production process, said layer having a thickness of 30 nm or less and a photoactivity of greater than 5.times.10.sup.-3 cm.sup.-1 min.sup.-1, by contacting the surface of the ribbon with a gaseous mixture comprising a source of titanium while the ribbon is at a temperature of from 625.degree. to 720.degree. C. 2. A process as claimed in claim 1 wherein the ribbon is at a temperature in the range 670.degree. C. to 720.degree. C. 3. A process as claimed in claim 1 wherein the gaseous mixture comprises titanium tetraethoxide as the source of titanium. 4. A process as claimed in claim 1 wherein the gaseous mixture comprises titanium chloride as the source of titanium and an ester other than a methyl ester. 5. A process as claimed in claim 4 wherein the ester comprises an alkyl ester having an alkyl group with a .beta. hydrogen. 6. A process as claimed in claim 4 wherein the ester comprises a carboxylate ester. 7. A process as claimed in claim 4 wherein the ester is an alkyl ester having a C.sub.2 to C.sub.4 alkyl group. 8. A process as claimed in claim 7 wherein the ester comprises an ethyl ester. 9. A process as claimed in claim 8 wherein the ester comprises ethyl acetate. 10. A process as claimed in claimed in claim 4 wherein the ester is the only source of oxygen in the fluid mixture. 11. A process as claimed in claim 1 wherein the process is performed at substantially atmospheric pressure. Description BACKGROUND OF THE INVENTION This invention relates to a process for producing photocatalytically active coated substrates, in particular, but not exclusively, it relates to a process for producing photocatalytically active coated glass and such coated glass. It is known to deposit thin coatings having one or more layers, with a variety of properties, on to substrates including on to glass substrates. One property of interest is photocatalytic activity which arises by the photogeneration, in a semiconductor, of a hole-electron pair when the semiconductor is illuminated by light of a particular frequency. The hole-electron pair can be generated in sunlight and can react in humid air to form hydroxy and peroxy radicals on the surface of the semiconductor. The radicals oxidise organic grime on the surface. This property has an application in self-cleaning substrates, especially in self-cleaning glass for windows. Titanium dioxide may be an efficient photocatalyst and may be deposited on to substrates to form a transparent coating with photocatalytic self-cleaning properties. Titanium oxide photocatalytic coatings are disclosed in EP 0 901 991 A2, WO 97/07069, WO 97/10186, WO 98/41480, in Abstract 735 of 187th Electrochemical Society Meeting (Reno, Nev., 95-1, p.1102) and in New Scientist magazine (26 Aug. 1995, p.19). In WO 98/06675 a chemical vapour deposition process is described for depositing titanium oxide coatings on hot flat glass at high deposition rate using a precursor gas mixture of titanium chloride and an organic compound as source of oxygen for formation of the titanium oxide coating. It has been thought that relatively thick titanium oxide coatings need to be deposited to provide good photocatalytic activity. For example, in WO 98/41480 it is stated that a photocatalytically active self-cleaning coating must be sufficiently thick so as to provide an acceptable level of activity and it is preferred that such a coating is at least about 200 .ANG. and more preferably at least about 500 .ANG. thick (the measured thickness of the titanium oxide coatings produced in the Examples all being in the range 400 .ANG. to 2100 .ANG.). However, a problem of relatively thick titanium oxide coatings is high visible light reflection and thus relatively low visible light transmission. This problem is recognised in the article in New Scientist magazine in relation to coated windscreens, where it is suggested that to reduce the effect of high reflection, dashboards might have to be coated in black velvet or some other material that does not reflect light into a coated windscreen. EP 0 901 991A2 referred to above, relates to photocatalytic glass panes with a coating of titanium oxide of a particular crystal structure characterised by the presence of particular peaks in its X-ray diffraction pattern. The specification contemplates a range of coating thickness (with the specific Examples all having thickness in the range 20 nm to 135 nm, the thinner coatings being less photocatalytically active than the thicker coatings). The specification also contemplates a range of deposition temperatures from as low as 300.degree. C. to as high as 750.degree. C., but prefers temperatures in the range 400.degree. C. to 600.degree. C., and in all the specific Examples of the invention the titanium dioxide layer is deposited at a temperature in or below this preferred range. The applicants have now found that by depositing the titanium oxide coatings at higher temperatures, especially temperatures above 600.degree. C., they are able to achieve coatings with an enhanced photocatalytic activity for a given thickness, enabling the same photocatalytic performance to be achieved with thinner coatings. Such thinner coatings tend to have, advantageously, lower visible light reflection and, apparently in consequence of their higher deposition temperature, improved durability, especially to abrasion and temperature cycling in a humid atmosphere. SUMMARY OF THE INVENTION The present invention accordingly provides a process for the production of a photocatalytically active coated substrate which comprises depositing a titanium oxide coating on the surface of a substrate by contacting the surface of the substrate with a fluid mixture containing a source of titanium and a source of oxygen, said substrate being at a temperature of at least 600.degree. C., whereby the coated surface of the substrate has a photocatalytic activity of greater than 5.times.10.sup.-3 cm.sup.-1 min.sup.-1 and a visible light reflection measured on the coated side of 35% or lower. Preferably, the substrate is at a temperature in the range 625.degree. C. to 720.degree. C., more preferably the substrate is at a temperature in the range 645.degree. C. to 720.degree. C. Advantageously, the fluid mixture comprises titanium chloride as the source of titanium and an ester other than a methyl ester. Thus, in a preferred embodiment, the present invention provides a process for the production of a photocatalytically active coated substrate which comprises depositing a titanium oxide coating having a thickness of less than 40 nm on a substrate by contacting a surface of the substrate with a fluid mixture comprising titanium chloride and an ester other than a methyl ester. The process may be performed wherein the surface of the substrate is contacted with the fluid mixture when the substrate is at a temperature in the range 600.degree. C. to 750.degree. C. Preferably, the ester is an alkyl ester having an alkyl group with a .beta. hydrogen (the alkyl group of an alkyl ester is the group derived from the alcohol in synthesis of an ester and a .beta. hydrogen is a hydrogen bonded to a carbon atom .beta. to the oxygen of the ether linkage in an ester). Preferably the ester is a carboxylate ester. Suitable esters may be alkyl esters having a C.sub.2 to C.sub.10 alkyl group, but preferably the ester is an alkyl ester having a C.sub.2 to C.sub.4 alkyl group. Preferably, the ester is a compound of formula: R--C(O)--O--C(X)(X')--C(Y)(Y')--R', where R and R' represent hydrogen or an alkyl group, X, X', Y and Y' represent monovalent substituents, preferably alkyl groups or hydrogen atoms and wherein at least one of Y and Y' represents hydrogen. Suitable esters that may be used in the process of the present invention include: ethyl formate, ethyl acetate, ethyl propionate, ethyl butyrate, n-propyl formate, n-propyl acetate, n-propyl propionate, n-propyl butyrate, isopropyl formate, isopropyl acetate, isopropyl propionate, isopropyl butyrate, n-butyl formate, n-butyl acetate and t-butyl acetate. Preferably, the ester comprises an ethyl ester, more preferably the ester comprises ethyl formate, ethyl acetate or ethyl propionate. Most preferably the ester comprises ethyl acetate. The fluid mixture may be in the form of a liquid, especially dispersed as a fine spray (a process often referred to as spray deposition), but preferably the fluid mixture is a gaseous mixture. A deposition process performed using a gaseous mixture as precursor is often referred to as chemical vapour deposition (CVD). The preferred form of CVD is laminar flow CVD, although turbulent flow CVD may also be used. The process may be performed on substrates of various dimensions including on sheet substrates, especially on cut sheets of glass, or preferably on-line during the float glass production process on a continuous ribbon of glass. Thus, preferably, the process is performed on-line during the float glass production process and the substrate is a glass ribbon. If the process is performed on line, it is preferably performed on the glass ribbon whilst it is in the float bath. An advantage of performing the process on-line is that coatings deposited on-line tend to be durable and in particular to have good abrasion and chemical resistance. An on-line deposition process is preferably, and other deposition processes may be, performed at substantially atmospheric pressure. In a particularly preferred embodiment there is provided a process for the production of a durable photocatalytically active coated glass which comprises depositing on the surface of a glass substrate a photocatalytically active titanium oxide layer by contacting the surface of the substrate, which is at a temperature in the range 645.degree. C. to 720.degree. C., preferably in the range 670.degree. C. to 720.degree. C. with a fluid mixture containing a source of titanium. As noted above, the applicants have found that by depositing the titanium oxide at high temperature, a coating of relatively high photocatalytic activity for its thickness may be produced and, as coatings of reduced thickness tend to have lower reflection, the invention also provides novel products having an advantageous combination of high photocatalytic activity with moderate or low light reflection. Thus, the present invention, in another aspect, provides a photocatalytically active coated substrate comprising a substrate having a photocatalytically active titanium oxide coating on one surface thereof, characterised in that the coated surface of the substrate has a photocatalytic activity of greater than 5.times.10.sup.-3 cm.sup.-1 min.sup.-1 and in that the coated substrate has a visible light reflection measured on the coated side of 35% or lower. High photocatalytic activity is advantageous because the amount of contaminants (including dirt) on the coated surface of the photocatalytically active coated substrate will be reduced quicker than on substrates with relatively low photocatalytic activity. Also, relatively quick removal of surface contaminants will tend to occur at low levels of UV light intensity. Photocatalytic activity for the purposes of this specification is determined by measuring the rate of decrease of the integrated absorbance of the infra-red absorption peaks corresponding to the C--H stretches of a thin film of stearic acid, formed on the coated substrate, under illumination by UV light from a UVA lamp having an intensity of about 32 W/m.sup.2 at the surface of the coated substrate and a peak wavelength of 351 nm. The stearic acid may be formed on the coated substrate by spin casting a solution of stearic acid in methanol as described below. Preferably, the coated surface of the substrate has a photocatalytic activity of greater than 1.times.10.sup.-2 cm.sup.-1 min.sup.-1, more preferably of greater than 3.times.10.sup.-2 cm.sup.-1 min.sup.-1. Low visible light reflection is advantageous because it is less distracting than high reflection and, especially for glass substrates, low visible light reflection corresponds to high visible transmission which is often required in architectural and especially automotive applications of glass. Preferably, the coated substrate has a visible reflection measured on the coated side of 20% or lower more preferably of 17% or lower and most preferably of 15% or lower. In most embodiments of the invention the substrate will be substantially transparent and in a preferred embodiment of the invention the substrate comprises a glass substrate. Usually the glass substrate will be a soda lime glass substrate. Where the substrate is a soda lime glass substrate or other alkali metal ion containing substrate, the coated substrate preferably has an alkali metal ion blocking underlayer between the surface of the substrate and the photocatalytically active titanium oxide coating. This reduces the tendency for alkali metal ions from the substrate to migrate into the photocatalytically active titanium oxide coating which is advantageous because of the well known tendency of alkali metal ions to poison semiconductor oxide coatings, reducing their activity. The alkali metal ion blocking underlayer may comprise a metal oxide but preferably the alkali metal ion blocking layer is a layer of silicon oxide. The silicon oxide may be silica but will not necessarily be stoichiometric and may comprise impurities such as carbon (often referred to as silicon oxycarbide and deposited as described in GB 2,199,848B) or nitrogen (often referred to as silicon oxynitride). It is advantageous if the alkali metal ion blocking underlayer is thin so that it has no significant effect on the optical properties of the coating, especially by reducing the transparency of a transparent coated substrate or causing interference colours in reflection or transmission. The suitable thickness range will depend on the properties of the material used to form the alkali metal ion blocking layer (especially its refractive index), but usually the alkali metal ion blocking underlayer has a thickness of less than 60 nm and preferably has a thickness of less than 40 nm. Where present, the alkali metal ion blocking underlayer should always be thick enough to reduce or block migration of alkali metal ions from the glass into the titanium oxide coating. An advantage of the present invention is that the photocatalytically active titanium oxide coating is thin (contributing to the low visible reflection of the coated substrate) but the coated substrate still has excellent photocatalytic activity. Preferably, the titanium oxide coating has a thickness of 30 nm or lower, more preferably the titanium oxide coating has a thickness of 20 nm or lower and most preferably the titanium oxide coating has a thickness in the range 2 nm to about 20 nm. The present invention is also advantageous because depositing thin titanium oxide coatings requires less precursor and the layers can be deposited in a relatively short time. A thin titanium oxide coating is also less likely to cause interference colours in reflection or transmission. However, a particular advantage is that the visible light reflection of a thin titanium oxide coating is low which is especially important when the coated substrate is coated glass. Usually the required visible light transmission of the coated glass will determine the thickness of the titanium oxide coating. Preferably, the coated surface of the substrate has a static water contact angle of 20.degree. or lower. Freshly prepared or cleaned glass has a hydrophilic surface (a static water contact angle of lower than about 40.degree. indicates a hydrophilic surface), but organic contaminants rapidly adhere to the surface increasing the contact angle. A particular benefit of coated substrates (and especially coated glasses) of the present invention is that even if the coated surface is soiled, irradiation of the coated surface by UV light of the right wavelength will reduce the contact angle by reducing or destroying those contaminants. A further advantage is that water will spread out over the low contact angle surface reducing the distracting effect of droplets of water on the surface (e.g. from rain) and tending to wash away any grime or other contaminants that have not been destroyed by the photocatalytic activity of the surface. The static water contact angle is the angle subtended by the meniscus of a water droplet on a glass surface and may be determined in a known manner by measuring the diameter of a water droplet of known volume on a glass surface and calculated using an iterative procedure. Preferably, the coated substrate has a haze of 1% or lower, which is beneficial because this allows clarity of view through a transparent coated substrate. In preferred embodiments, the coated surface of the substrate is durable to abrasion, such that the coated surface remains photocatalytically active after it has been subjected to 300 strokes of the European standard abrasion test. Preferably, the coated surface remains photocatalytically active after it has been subjected to 500 strokes of the European standard abrasion test, and more preferably the coated surface remains photocatalytically active after it has been subjected to 1000 strokes of the European standard abrasion test. This is advantageous because self-cleaning coated substrates of the present invention will often be used with the coated surface exposed to the outside (e.g. coated glasses with the coated surface of the glass as the outer surface of a window) where the coating is vulnerable to abrasion. The European standard abrasion test refers to the abrasion test described in European standard BS EN 1096 Part 2 (1999) and comprises the reciprocation of a felt pad at a set speed and pressure over the surface of the sample. In the present specification, a coated substrate is considered to remain photocatalytically active if, after being subjected to the European abrasion test, irradiation by UV light (e.g. of peak wavelength 351 nm) reduces the static water contact angle to below 15.degree.. To achieve this contact angle after abrasion of the coated substrate will usually take less than 48 hours of irradiation at an intensity of about 32 W/m.sup.2 at the surface of the coated substrate. Preferably, the haze of the coated substrate is 2% or lower after being subjected to the European standard abrasion test. Durable coated substrates according to the present invention may also be durable to humidity cycling (which is intended to have a similar effect to weathering). Thus, in preferred embodiments of the invention, the coated surface of the substrate is durable to humidity cycling such that the coated surface remains photocatalytically active after the coated substrate has been subjected to 200 cycles of the humidity cycling test. In the present specification, the humidity cycling test refers to a test wherein the coating is subjected to a temperature cycle of 35.degree. C. to 75.degree. C. to 35.degree. C. in 4 hours at near 100% relative humidity. The coated substrate is considered to remain photocatalytically active, if, after the test, irradiation by UV light reduces the static water contact angle to below 15.degree.. In a further preferred embodiment, the present invention provides a durable photocatalytically active coated glass comprising a glass substrate having a coating on one surface thereof, said coating comprising an alkali metal ion blocking underlayer and a photocatalytically active titanium oxide layer, wherein the coated surface of the substrate is durable to abrasion such that the coated surface remains photocatalytically active after it has been subjected to 300 strokes of the European standard abrasion test. In this embodiment, the coated glass preferably has a visible light reflection measured on the coated side of 35% or lower, and the photocatalytically active titanium oxide layer preferably has a thickness of 30 nm or lower. Thin coatings are durable to abrasion which is surprising because previously it has been thought that only relatively thick coatings would have good durability. In a still further embodiment, the present invention provides a coated glass comprising a glass substrate having a photocatalytically active titanium oxide coating on one surface thereof, characterised in that the coated surface of the glass has a photocatalytic activity of greater than 4.times.10.sup.-2 cm.sup.-1 min.sup.-1, preferably greater than 6.times.10.sup.-2 cm.sup.-1 min.sup.-1 and more preferably greater than 8.times.10.sup.-2 cm.sup.-1 min.sup.-1 and in that the coated glass has a visible light reflection measured on the coated side of less than 20%. Coated substrates according to the present invention have uses in many areas, for example as glazings in windows including in a multiple glazing unit comprising a first glazing pane of a coated substrate in spaced opposed relationship to a second glazing pane, or, when the coated substrate is coated glass, as laminated glass comprising a first glass ply of the coated glass, a polymer interlayer (of, for example, polyvinylbutyral) and a second glass ply. In addition to uses in self-cleaning substrates (especially self-cleaning glass for windows), coated substrates of the present invention may also be useful in reducing the concentration of atmospheric contaminants. For example, coated glass under irradiation by light of UV wavelengths (including UV wavelengths present in sunlight) may destroy atmospheric contaminants for example, nitrogen oxides, ozone and organic pollutants, adsorbed on the coated surface of the glass. This use is particularly advantageous in the open in built-up areas (for example, in city streets) where the concentration of organic contaminants may be relatively high (especially in intense sunlight), but where the available surface area of glass is also relatively high. Alternatively, the coated glass (with the coated surface on the inside) may be used to reduce the concentration of atmospheric contaminants inside buildings, especially in office buildings having a relatively high concentration of atmospheric contaminants. The invention is illustrated but not limited by the following drawings. BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 is a graph of photocatalytic activity of coated glass produced by a process according to the invention as a function of the thickness of the titanium oxide layer. FIG. 2 illustrates apparatus for on line chemical vapour deposition of coatings according to the invention. DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS In FIG. 1 the coated glasses were produced using an on-line CVD process as described in the Examples, below. The open circles 1 relate to titanium oxide layers deposited using titanium tetrachloride as titanium precursor, and the crosses 2 relate to titanium oxide layers deposited using titanium tetraethoxide as titanium precursor. The layers of the coating may be applied on line onto the glass substrate by chemical vapour deposition during the glass manufacturing process. FIG. 2 illustrates an apparatus, indicated generally at 10, useful for the on line production of the coated glass article of the present invention, comprising a float section 11, a lehr 12, and a cooling section 13. The float section 11 has a bottom 14 which contains a molten tin bath 15, a roof 16, sidewalls (not shown), and end walls 17, which together form a seal such that there is provided an enclosed zone 18, wherein a non-oxidising atmosphere is maintained to prevent oxidation of the tin bath 15. During operation of the apparatus 10, molten glass 19 is cast onto a hearth 20, and flows therefrom under a metering wall 21, then downwardly onto the surface of the tin bath 15, forming a float glass ribbon 37, which is removed by lift-out rolls 22 and conveyed through the lehr 12, and thereafter through the cooling section 13. A non-oxidising atmosphere is maintained in the float section 11 by introducing a suitable gas, such as for example one comprising nitrogen and 2% by volume hydrogen, into the zone 18, through conduits 23 which are operably connected to a manifold 24. The non-oxidizing gas is introduced into the zone 18 from the conduits 23 at a rate sufficient to compensate for losses of the gas (some of the non-oxidizing atmosphere leaves the zone 18 by flowing under the end walls 17), and to maintain a slight positive pressure above ambient pressure. The tin bath 15 and the enclosed zone 18 are heated by radiant heat directed downwardly from heaters 25. The heat zone 18 is generally maintained at a temperature of about 1330.degree. F. to 1400.degree. F. (721.degree. C. to 760.degree. C.). The atmosphere in the lehr 12 is typically air, and the cooling section 13 is not enclosed. Ambient air is blown onto the glass by fans 26. The apparatus 10 also includes coaters 27, 28, 29 and 30 located in series in the float zone 11 above the float glass ribbon 37. The precursor gaseous mixtures for the individual layers of the coating are supplied to the respective coaters, which in turn direct the precursor gaseous mixtures to the hot surface of the float glass ribbon 37. The temperature of the float glass ribbon 37 is highest at the location of the coater 27 nearest the hearth 20 and lowest at the location of the coater 30 nearest the lehr 12. The invention is further illustrated by the following Examples, in which coatings were applied by laminar flow chemical vapour deposition in the float bath on to a moving ribbon of float glass during the glass production process. In the Examples two layer coatings were applied to the glass ribbon. All gas volumes are measured at standard temperature and pressure unless otherwise stated. The thickness values quoted for the layers were determined using high resolution scanning electron microscopy and optical modelling of the reflection and transmission spectra of the coated glass. Thickness of the coatings was measured with an uncertainty of about 5%. The transmission and reflection properties of the coated glasses were determined using an Hitachi U-4000 spectrophotometer. The a, b and L* values mentioned herein of the transmission and/or reflection colour of the glasses refer to the CIE Lab colours. The visible reflection and visible transmission of the coated glasses were determined using the D65 illuminant and the standard CIE 2.degree. observer in accordance with the ISO 9050 standard (Parry Moon airmass 2) The haze of the coated glasses was measured using a WYK-Gardner Hazeguard+haze meter. The photocatalytic activity of the coated glasses was determined from the rate of decrease of the area of the infrared peaks corresponding to C--H stretches of a stearic acid film on the coated surface of the glass under illumination by UVA light. The stearic acid film was formed on samples of the glasses, 7-8 cm square, by spin casting 20 .mu.l of a solution of stearic acid in methanol (8.8.times.10.sup.-3 mol dm.sup.-3) on the coated surface of the glass at 2000 rpm for 1 minute. Infra red spectra were measured in transmission, and the peak height of the peak corresponding to the C--H stretches (at about 2700 to 3000 cm.sup.-1) of the stearic acid film was measured and the corresponding peak area determined from a calibration curve of peak area against peak height. The coated side of the glass was illuminated with a UVA-351 lamp (obtained from the Q-Panel Co., Cleveland, Ohio, USA) having a peak wavelength of 351 nm and an intensity at the surface of the coated glass of approximately 32 W/m.sup.2. The photocatalytic activity is expressed in this specification either as the rate of decrease of the area of the IR peaks (in units of cm.sup.-1 min.sup.-1) or as t.sub.90% (in units of min) which is the time of UV exposure taken to reduce the peak height (absorption) of a peak in the wavelength area down to 10% of its initial value. The static water contact angle of the coated glasses was determined by measuring the diameter of a water droplet (volume in the range 1 to 5 .mu.l) placed on the surface of the coated glass after irradiation of the coated glass using the UVA 351 lamp for about 2 hours (or as otherwise specified). EXAMPLES 1-15 A ribbon of 1 mm thick soda lime float glass advancing at a lehr speed of 300 m/hour was coated with a two-layer coating as the ribbon advanced over the float bath at a position where the glass temperature was in the range of about 650.degree. C. to about 670.degree. C. The float bath atmosphere comprised a flowing gaseous mixture of nitrogen and 9% hydrogen at a bath pressure of approximately 0.15 mbar. Layer 1 (the first layer to be deposited on the glass) was a layer of silicon oxide. Layer 1 was deposited by causing a gaseous mixture of monosilane (SiH.sub.4, 60 ml/min), oxygen (120 ml/min), ethylene (360 ml/min) and nitrogen (8 litres/min) to contact and flow parallel to the glass surface in the direction of movement of the glass using coating apparatus as described in GB patent specification 1 507 966 (referring in particular to FIG. 2 and the corresponding description on page 3 line 73 to page 4 line 75) with a path of travel of the gaseous mixture over the glass surface of approximately 0.15 m. Extraction was at approximately 0.9 to 1.2 mbar. The glass ribbon was coated across a width of approximately 10 cm at a point where its temperature was approximately 670.degree. C. The thickness of the silica layer was about 20 to 25 m. Layer 2 (the second layer to be deposited) was a layer of titanium dioxide. Layer 2 was deposited by combining separate gas streams comprising titanium tetrachloride in flowing nitrogen carrier gas, ethyl acetate in flowing nitrogen carrier gas and a bulk flow of nitrogen of 8 l/min (flow rate measured at 20 psi) into a gaseous mixture and then delivering (through lines maintained at about 250.degree. C.) the gaseous mixture to coating apparatus consisting of an oil cooled dual flow coater. The pressure of the nitrogen carrier and bulk nitrogen gases was approximately 20 pounds per square inch. The gaseous mixture contacted and flowed parallel to the glass surface both upstream and downstream along the glass ribbon. The path of travel of the gaseous mixture downstream was about 0.15 m and upstream was about 0.15 m with extraction of about 0.15 mbar. Titanium tetrachloride and ethyl acetate were entrained in separate streams of flowing nitrogen carrier gas by passing nitrogen through bubblers containing either titanium tetrachloride or ethyl acetate. The flow rates of the nitrogen carrier gases are described in Table 1 (the flow rates were measured at 20 psi). The titanium tetrachloride bubbler was maintained at a temperature of 69.degree. C. and the ethyl acetate bubbler was maintained at a temperature of 42.degree. C. The estimated flow rates of entrained titanium tetrachloride and entrained ethyl acetate are also described in Table 1 for each of the Examples 1 to 15. The properties of the two-layer coatings were measured. Values of the thickness of layer 2 (the titanium oxide layer), and values of the visible reflection measured on the coated side, L* and haze of the coated glasses are described in Table 2 for the Examples 1-15. The haze of each coated glass was below 0.2%. The photocatalytic activity and static water contact angle of the coated glasses were determined. The initial peak height and initial peak area of the IR peaks corresponding to the stearic acid C--H stretches, the photocatalytic activity, the static water contact angle and t.sub.90% for the Examples 1-15 are described in Table 3. The thickness of the titanium oxide layer, surprisingly has little effect on photocatalytic activity. EXAMPLES 16-19 Examples 16-19 were conducted under the same conditions as Examples 1-15 except that the bath pressure was approximately 0.11 mbar, extraction for deposition of the silica undercoat (layer 1) was approximately 0.7 mbar, the titanium tetrachloride bubbler was maintained at a temperature of approximately 100.degree. C., the ethyl acetate bubbler was maintained at a temperature of approximately 45.degree. C. and the delivery lines were maintained at a temperature of approximately 220.degree. C. The flow rates of nitrogen carrier gas, and the estimated flow rates of entrained titanium tetrachloride and entrained ethyl acetate are disclosed for each of the examples 16-19 in Table 1. Values of the estimated thickness of layer 2 (the titanium oxide layer), and values of visible reflection measured on the coated side, L* and haze of the coated glasses are described in Table 2 for each of the Examples 16-19. The initial peak height and initial peak area of the IR peaks corresponding to the stearic acid C--H stretches, the photocatalytic activity, t.sub.90% and the static water contact angle and for each of the Examples 16-19 are described in Table 3. The photocatalytic activity of the Examples 16-19 was not substantially greater than that of the Examples 1-15 despite the thicker titanium oxide (and hence more reflective) coatings. TABLE 1 Nitrogen Carrier Gas Example Flow Rates to Bubblers (l/min, measured at 20 psi) TiCl.sub.4 Ethyl Acetate Ethyl Acetate flow rate flow rate TiCl.sub.4 Bubbler Bubbler (l/min) (l/min) 1 2 0.16 0.12 1 0.3 0.032 0.024 0.46 0.14 3 4 5 6 7 8 9 10 11 12 0.12 0.08 0.12 0.12 0.08 0.08 0.04 0.04 0.04 0.16 0.45 0.2 0.15 0.75 0.3 0.5 0.1 0.15 0.25 0.1 0.024 0.016 0.024 0.024 0.016 0.016 0.008 0.008 0.008 0.032 0.21 0.09 0.07 0.35 0.14 0.23 0.05 0.07 0.12 0.05 13 14 15 16 17 18 19 0.08 0.16 0.16 0.1 0.08 0.06 0.04 0.1 0.4 0.2 0.5 0.4 0.3 0.2 0.016 0.032 0.032 0.088 0.070 0.053 0.035 0.05 0.19 0.09 0.27 0.22 0.16 0.11 TABLE 2 Example 1 Thickness of Visible reflection titanium oxide of coated glass L* value of Haze layer (nm) (%) coated glass (%) (%) 15 14.1 44 0.12 2 14.3 13.9 44 3 14.2 13.2 43 4 11.3 11.4 40 5 12.1 12.1 41 6 11.0 a a 7 8 a a 8 7.2 9.7 37 9 6.1 9.1 36 10 5.6 9 36 11 4.6 8.7 35 12 13 15.6 16.0 15.4 a 46 a 14 17.5 16.2 47 15 16 17 20.3 a ca 68 19.5 28.4 29.1 51 47.8 58.4 0.07 0.12 0.08 0.08 0.07 0.11 0.04 0.05 0.07 0.06 0.1 0.13 0.14 0.37 0.1 0.3 18 ca 32 25.9 55.6 19 ca 27 a not measured 20.5 50.2 0.24 0.2 TABLE 3 IR Peaks corresponding to stearic acid film C-H stretches (2700-3000 cm.sup.-1) PhotoStatic Initial Peak Initial catalytic Water cm.sup.-1 Height (arbitrary Peak Area units) (cm.sup.-1) Activity Contact (.times.10.sup.-2 Angle t.sub.90% Example min.sup.-1) (.degree.) (min) 1 2 3 4 0.030 0.0331 0.0311 0.0324 1.04 1.15 1.08 1.13 9.4 10.4 12.2 6.8 17 15 13 14 .+-. .+-. .+-. .+-. 5 1 2 1 10 10 8 15 5 6 7 8 9 10 11 12 13 0.0287 0.028 0.0343 0.0289 0.0289 0.0278 0.0344 0.0291 0.0289 1.00 0.98 1.20 1.03 1.01 0.97 1.20 1.02 1.01 8.2 8.8 10.8 6.6 6.5 6.2 5.4 10.2 9.1 16 15 15 16 14 18 18 12 14 .+-. .+-. .+-. .+-. .+-. .+-. .+-. .+-. .+-. 3 1 1 1 2 2 1 1 2 11 10 10 14 14 14 20 9 10 14 15 16 17 18 19 0.0269 0.0331 0.0227 0.026 0.0225 0.0258 0.94 1.15 0.79 0.91 0.79 0.90 9.4 8.7 17.8 10.2 10.1 10.1 15 .+-. 2 15 .+-. 2 12 12 13 16 9 12 4 8 7 8 EXAMPLES 20-27 The Examples 20-27 were conducted under the same conditions as Examples 1-15 except that layer 2 was deposited from a gaseous mixture comprising titanium tetraethoxide entrained in nitrogen carrier gas by passing the carrier gas through a bubbler containing titanium tetraethoxide maintained at a temperature of 170.degree. C. The flow rates of nitrogen carrier gas (measured at 20 psi) and titanium tetraethoxide are described in Table 4 for each of the Examples 20-27. The flow rate of bulk nitrogen gas was 8.5 l/min (measured at 20 psi). The properties of the two-layer coatings were measured. Values of the thickness of layer 2 (the titanium oxide layer), and values of the visible reflection measured on the coated side and haze of the coated glasses are described in Table 5 for the Examples 20-27. The haze of each coated glass was below 0.7%. The photocatalytic activity and static water contact angle of the coated glasses were determined. The initial peak height and initial peak area of the IR peaks corresponding to the stearic acid C--H stretches, the photocatalytic activity and t.sub.90%, and the static water contact angle for each of the Examples 20-27 are described in Table 6. EXAMPLES 28 AND 29 The Examples 28 and 29 were conducted under the same conditions as Examples 20-27 except that the titanium tetraethoxide bubbler was maintained at a temperature of 168.degree. C. and the bath pressure was 0.11 mbar. Data relating to Examples 28-29 equivalent to data for Examples 20-27 are described in Tables 4, 5 and 6. TABLE 4 Nitrogen Carrier Gas Flow Rates to Example 20 21 22 23 24 25 Titanium tetraethoxide bubbler Titanium ethoxide flow (l/min, measured at 20 psi) rate (l/min) 0.25 0.014 0.15 0.008 0.2 0.011 0.25 0.014 0.3 0.017 0.35 0.019 26 0.2 0.011 27 28 29 0.1 0.6 0.4 0.006 0.030 0.020 TABLE 5 Example 20 21 Thickness of titanium Visible reflection of Haze oxide layer (nm) coated glass (%) (%) 13 a 0.4 13 a 0.29 22 23 24 a 25 26 27 28 29 Not measured 16 18 24 15.7 a a 0.29 0.28 a 26 9.9 4.7 38.3 31.9 a 10.9 8.8 35.2 28.4 0.61 0.19 0.29 0.29 0.22 TABLE 6 IR Peaks corresponding to stearic acid film C-H stretches (2700-3000 cm.sup.-1) PhotoStatic Initial Peak Initial catalytic Water Height Peak Activity Contact (arbitrary Area (.times.10.sup.-2 cm.sup.-1 Angle t.sub.90% Example units) (cm.sup.-1) 0.027 0.031 0.024 0.030 0.029 0.953 1.095 0.838 1.029 1.015 min.sup.-1) (.degree.) (min) 20 21 22 23 24 5.7 5.7 3.6 7.1 7 19 .+-. a 15 .+-. 11 .+-. 17 .+-. 5 15 17 2 3 3 21 13 13 25 a 0.031 1.071 7.4 13 .+-. 4 13 26 0.031 27 0.029 28 0.021 29 0.024 Not measured 1.085 0.998 0.733 0.848 4.4 3.2 3.6 3.3 21 .+-. 3 22 16 .+-. 5 28 13 18 14 23 EXAMPLES 30-42 In Examples 30 to 42, two-layer coatings were applied by on line CVD to a float glass ribbon across its full width of approximately 132 inches (3.35 m) in the float bath during the float glass production process. The apparatus used to deposit the coating is illustrated in FIG. 2. The float bath atmosphere comprised nitrogen and 2% by volume hydrogen. Bath pressure was 0.15 mbar. The two layer coating consisted of a silicon oxide layer deposited first on the float glass ribbon and titanium oxide layer deposited on to the silicon oxide layer. The precursor chemistry of the gaseous mixtures used to deposit the coating was the same as that used in Examples 1-15. The temperature of deposition of the layers was varied by using different coaters 27, 28, 29 or 30 (referring to FIG. 2). Coater 27 located nearest the hearth being hottest and coater 30 located nearest the lehr being coolest. In Examples 30-33 and 42 two coaters (28 and 29 in Examples 30-33 and coaters 27 and 28 in Example 42) were used to deposit the silicon oxide coating. The benefit of using two coaters to deposit the silicon oxide layer is that longer production run times are possible. The gaseous mixture used to deposit the silicon oxide layer for Examples 30 to 41 consisted of the following gases at the following flow rates: helium (250 l/min), nitrogen (285 l/min), monosilane (2.5 l/min), ethylene (15 l/min) and oxygen (10 l/min). For Example 42, the same gases and flow rates were used except for monosilane (2.3 l/min), ethylene (13.8 l/min) and oxygen (9.2 l/min). Where two coaters were used to deposit the silicon oxide layer in Examples 30 to 42, the above flow rates were used for each coater. In Examples 30-42 the deposition temperatures (i.e. the temperature of the float glass ribbon under the coater corresponding to each of the coaters 27-30) was as indicated in Table 7. The temperatures in Table 7 have an uncertainty of about .+-.50.degree. F. (.+-.28.degree. C.). The extraction for each coater was at approximately 2 mbar. TABLE 7 Coater 27 28 29 30 Approx. Temperature of Glass Ribbon 1330.degree. F. (721.degree. C.) 1275.degree. F. (690.degree. C.) 1250.degree. F. (677.degree. C.) 1150.degree. F. (621.degree. C.) Titanium tetrachloride (TiCl.sub.4) and ethyl acetate were entrained in separate nitrogen/helium carrier gas streams. For the evaporation of TiCl.sub.4 a thin film evaporator was used. The liquid TiCl.sub.4 was held in a pressurised container (head pressure approx 5 psi). This was used to deliver the liquid to a metering pump and Coriolis force flow measurement system. The metered flow of the precursor was then fed into a thin film evaporator at a temperature of 110.degree. F. (43.degree. C.). The TiCl.sub.4 was then entrained in the carrier gas (helium) and delivered to the mixing point down lines held at 250.degree. F. (121.degree. C.). The ethyl acetate was delivered in a similar way. The liquid ethyl acetate was held in a pressurised container (head pressure approx 5 psi). This was used to deliver the liquid to a metering pump and Coriolis force flow measurement system. The metered flow of the precursor was then fed into a thin film evaporator at a temperature of 268.degree. F. (131.degree. C.). The evaporated ethyl acetate was then entrained in the carrier gas (helium/nitrogen mixture) and delivered to the mixing point down lines held at approximately 250.degree. F. (121.degree. C.). The TiCl.sub.4 and ethyl acetate gas streams were combined to form the gaseous mixture used to deposit the titanium oxide layer. This mixing point was just prior to the coater. The line speed of the float glass ribbon, the temperature of deposition of the silicon oxide and temperature of deposition of the titanium oxide layers and the flow rates of the He/N.sub.2 bulk carrier gas and the flow rate of TiCl.sub.4 and ethyl acetate are described for Examples 30-42 in Table 8. The coated float glass ribbon was cooled and cut and the optical properties and photocatalytic activity of samples determined. Table 9 describes the haze, optical properties in transmission and reflection (visible percent transmission/reflection and colour co-ordinates using the LAB system) of the samples. The coated glasses were subjected to abrasion testing in accordance with BS EN 1096, in which a sample of size 300 mm.times.300 mm is fixed rigidly, at the four corners, to the test bed ensuring that no movement of the sample is possible. An unused felt pad cut to the dimensions stated in the standard (BS EN 1096 Part 2 (1999)) is then mounted in the test finger and the finger lowered to the glass surface. A load pressure on the test finger of 4N is then set and the test started. The finger is allowed to reciprocate across the sample for 500 strokes at a speed of 60 strokes/min .+-.6 strokes/min. Upon completion of this abrasion the sample is removed and inspected optically and in terms of photocatalytic activity. The sample is deemed to have passed the test if the abrasion results in a change in transmission of no more than .+-.5% when measured at 550 nm and the coated substrate remains photocatalytically active which means that, after the test irradiation by UV light for 2 hours reduces the static water contact angle to below 15.degree.. The glasses were also subjected to a humidity cycling test in which the coating is subjected to a temperature cycle of 35.degree. C. to 75.degree. C. to 35.degree. C. in 4 hours at near 100% relative humidity. The static water contact angle of the coated glasses as produced and after 130 minutes of UV irradiation (UVA 351 mm lamp at approximately 32 W/m.sup.2) and after 300, 500 and/or 1000 strokes of the European standard abrasion test described in Table 10. The contact angle of the abraded samples was determined after irradiation for 2 hours. The samples deposited at the higher temperatures of 1330-1250.degree. F. (721.degree. C. to 677.degree. C.) were photocatalytically active even after 1000 European standard abrasion strokes or after 200 humidity cycles. The photocatalytic activity in terms of t.sub.90% of the coated glasses as produced and after 300, 500 and/or 1000 strokes of the European standard abrasion test and after 200 humidity testing cycles are described in Table 11. In Table 11, the term Active indicates that the coated glasses were photocatalytically active but that t.sub.90% was not determined. TABLE 8 Titanium Oxide Layer Lineof Flow Silica layer Flow Rates Rates of Precursors Speed Deposition Deposition Carrier Gases TiCl.sub.4 Ethyl Acetate Example (m/min) Temperature/.degree. C. Temperature/.degree. C. He L/min N.sub.2 L/min cc/min cc/min 30 10.9 690 & 677 621 300 300 6.3 16.3 31 10.9 690 & 677 621 300 300 6.3 32 16.3 10.9 690 & 677 621 300 6.3 33 16.3 10.9 690 & 677 621 300 6.3 34 6 16.3 10.9 690 621 300 10.9 690 621 300 10.9 690 621 300 37 10.9 690 677 300 5.5 38 14.7 10.9 690 677 300 5.5 39 14.7 10.9 690 677 300 5.5 14.7 300 300 300 16 300 35 6 16 300 36 6 16 300 300 300 40 10.9 690 677 300 5.5 41 4 14.7 6.5 721 690 300 721 & 690 677 300 300 300 10.7 42 12.1 300 9.5 25.4 Example TABLE 9 Film Side Reflection R (%) L* a b 30 31 32 33 34 35 36 Transmission T (%) L* a Haze b (%) 14.2 14.6 14.6 13.8 13.6 13.8 12.9 44.5 45.1 45.1 44.0 43.7 43.9 42.6 0.3 -10.3 84.3 93.6 0.3 -10.4 84.5 93.7 0.3 -10.5 84.3 93.6 0.3 -9.8 85.5 94.1 0.1 -8.7 84.8 93.8 0.1 -8.8 85.4 94.1 0.1 -8.2 85.8 94.2 -1.2 -1.1 -1.1 -1.1 -1.1 -1.1 -1.1 3.6 3.4 3.6 2.9 2.7 2.6 2.5 0.11 0.30 0.12 0.15 0.12 0.11 0.14 37 12.6 38 11.9 39 11.5 40 11.6 41 a 42 14 a Not Measured 42.2 41.0 40.4 40.6 a 44.3 0.1 0.1 0.0 0.0 a 0.1 -1.1 -1.1 -1.1 -1.1 a -1.1 2.3 1.7 1.8 1.8 a 3.1 0.08 0.07 0.10 0.08 a 0.14 Example 30 31 32 -7.9 -6.9 -6.5 -6.6 a -9.9 86.1 87.1 87.2 86.9 a 84.8 94.4 94.8 94.8 94.7 a 93.8 TABLE 10 Static Water Contact Angle (.degree.) after Number of Abrasion Strokes 0 (after 0 irradiation 130 min UV) 300 500 1000 2.3 3.3 failed 2.0 3.2 failed a a failed 33 2.0 34 a 35 2.0 36 2.1 37 2.2 38 2.0 39 1.9 40 2.2 41 7.8 42 4.7-5.3 a Not measured 3.2 failed a 3.2 3.4 3.3 3.1 3.1 3.2 7.8 4.7-5.3 failed failed failed <15 <15 <15 <15 10.1 5.6-9.8 TABLE 11 Example 30 31 32 33 34 35 36 37 38 39 40 41 42 t.sub.90% (min) after Number of Abrasion Strokes 0 300 500 1000 7.5 failed 18.5 failed 8.5 failed 8 failed 21 failed 4 failed 8.5 failed 15.5 Ca. 2160 18.5 Ca. 2160 17 Ca. 2160 18.5 Ca. 2160 a 45 ca. 2160 2800 t.sub.90% (min) after 200 Humidity Cycles failed failed failed failed failed failed failed Active Active Active Active Active Active a Not measured (3 United States Patent Hayakawa , et al. of 4) 6,830,785 December 14, 2004 Method for photocatalytically rendering a surface of a substrate superhydrophilic, a substrate with a superhydrophilic photocatalytic surface, and method of making thereof Abstract The surface of a substrate is coated with an abrasion-resistant photocatalytic coating comprised of a semiconductor photocatalyst. upon irradiation by a light having a wavelength of an energy higher than the bandgap energy of the photocatalyst, water is chemisorbed onto the surface in the form of hydroxyl groups (OH.sup.-) whereby the surface of the photocatalytic coating is rendered highly hydrophilic. In certain embodiments, the surface of a mirror, lens, or windowpane is coated with the photocatalytic coating to exhibit a high degree of antifogging function. In another embodiment, an article or product coated with the photocatalytic coating is disposed outdoors and the highly hydrophilic surface thereof is self-cleaned as it is subjected to rainfall. In a still another embodiment, an article is coated with the photocatalytic coating and, when the article is soaked in, rinsed by or wetted with water, fatty dirt and contaminants are readily released without resort to a detergent. Inventors: Hayakawa; Makoto (Kita-kyushu, JP); Kojima; Eiichi (Kita-kyushu, JP); Norimoto; Keiichiro (Kita-kyushu, JP); Machida; Mitsuyoshi (Kita-kyushu, JP); Kitamura; Atsushi (Kita-kyushu, JP); Watanabe; Toshiya (Kita-kyushu, JP); Chikuni; Makoto (Kita-kyushu, JP); Fujishima; Akira (Kawasaki, JP); Hashimoto; Kazuhito (Yokohama, JP) Assignee: Toto Ltd. (Fukuoka-ken, JP) Appl. No.: 374344 Filed: August 13, 1999 Foreign Application Priority Data Mar 20, 1995[JP] 7/099425 Apr 06, 1995[JP] 7/117600 Jun 14, 1995[JP] 7/182019 Jun 14, 1995[JP] 7/182020 Jul 08, 1995[JP] 7/205019 Nov 09, 1995[JP] 7/326167 Dec 22, 1995[JP] 7/354649 427/553; 134/1 Current U.S. Class: B05D 003/06; B08B 003/00; B08B 007/00; B08B 017/00 Intern'l Class: 427/553,554,555,556,581 134/1 Field of Search: References Cited [Referenced By] U.S. Patent Documents 3347816 Oct., 1967 Krauss et al. 3451833 Jun., 1969 Bonitz et al. 3640712 Feb., 1972 Field et al. 3871881 Mar., 1975 Mikelsons. 3976497 Aug., 1976 Clark. 4661372 Apr., 1987 Mance 4954465 Sep., 1990 Kawashima et al. 4955208 Sep., 1990 Kawashima et al. 5547823 Aug., 1996 Murasawa et al. 5593737 Jan., 1997 Meinzer et al. 5594585 Jan., 1997 Komatsu. 5595813 Jan., 1997 Ogawa et al. 5616532 Apr., 1997 Heller et al. 5643436 Jul., 1997 Ogawa et al. 5755867 May., 1998 Chikuni et al. 5759251 Jun., 1998 Nakamura et al. 106/286. 5854708 Dec., 1998 Komatsu et al. 359/507. 5859086 Jan., 1999 Freund et al. 427/553. 5874125 Feb., 1999 Kanoh et al. 427/553. 5989787 Nov., 1999 Kanoh et al. 427/553. 6037289 Mar., 2000 Chopin et al. 427/169. 6045903 Apr., 2000 Seino et al. 427/165. 6055085 Apr., 2000 Nakashima et al. 359/241. 6071606 Jun., 2000 Yamazaki et al. 428/325. 427/553. 427/553. 6090489 Jul., 2000 Hayakawa et al. 427/301. 6103363 Aug., 2000 Boire et al. 427/164. 6165256 Dec., 2000 Hayakawa et al. 6165619 Dec., 2000 Ikenaga et al. 427/387. 6193378 Feb., 2001 Tonar et al. 359/603. 6194346 Feb., 2001 Tada et al. 502/224. 6335061 Jan., 2002 Kanamori et al. 427/515. 6637336 Oct., 2003 Suda et al. 101/465. 6673433 Jan., 2004 Saeki et al. 428/323. 6679978 Jan., 2004 Johnson et al. 427/585. 6680135 Jan., 2004 Boire et al. 428/702. 2003/0003304 Jan., 2003 Ohtsu 428/412. 2003/0027000 Feb., 2003 Greenberg et al. 427/255. 2003/0181329 Sep., 2003 Tanaka et al. 502/338. 2003/0207028 Nov., 2003 Boire et al. 427/226. 2003/0213391 Nov., 2003 Suda et al. 101/463. 2003/0220194 Nov., 2003 Sakatani et al. 502/350. 2004/0009361 Jan., 2004 Street et al. 428/482. 2004/0028911 Feb., 2004 Hurst et al. 427/255. Foreign Patent Documents 0433915 Jun., 1991 EP. 0590477 Apr., 1994 EP. 1022588 Jul., 2000 EP. 149281/78 Dec., 1978 JP. 61-83106 Apr., 1986 JP. 61-91042 May., 1986 JP. 63-100042 May., 1988 JP. 1218635 Aug., 1989 JP. 1-218635 Aug., 1989 JP. 1288321 Nov., 1989 JP. 3101926 Apr., 1991 JP. 4-174679 Jun., 1992 JP. 5-302173 Nov., 1993 JP. 106/13. 6298520 Oct., 1994 JP. 6315614 Nov., 1994 JP. 7051646 Feb., 1995 JP. 7113272 May., 1995 JP. 8119673 May., 1995 JP. 7171408 Jul., 1995 JP. 60-221702 Nov., 1995 JP. 8164334 Jun., 1996 JP. 8313705 Nov., 1996 JP. 9173783 Jul., 1997 JP. 9-173783 Jul., 1997 JP. 9-224793 Sep., 1997 JP. 9-227157 Sep., 1997 JP. 9227157 Sep., 1997 JP. 9-227158 Sep., 1997 JP. 9227158 Sep., 1997 JP. 9235140 Sep., 1997 JP. 9-235140 Sep., 1997 JP. 9241037 Sep., 1997 JP. 9-241037 Sep., 1997 JP. WO9511751 May., 1995 WO. Other References Derwent Abstract of CN 1421496 A, Jun. 4, 2003.* Highly Transparent and Photoactive TIO2 Thin Film Coated on Glass Substrate, S. Fukayama 1996 with Translation (date & month, not given or not Translated). Highly Transparent and Photoactive TIO2 Thin Film Coated on Glass Substrate, S. Fukayama, Mar. 20, 1995, The Electrochemical Society of Japan with Translation. Preparing Catalyst Using Metal Alkoxide (no date, no author) (In Translation or Apparent) with Translation partial. "Highly Transparent and Photoactive TIo2 Thin Film Coated on Glass Substrates" from the 187th Electrochemical Society meeting, Reno 21-26, May 1994 Extended Abstracts 95-1 (for abstract 735 p1102) Fukayama S, et al. Derwent Abstract (008031514) of Japanese Pat. # JP 1218635 A, Published Only 1.sup.st Page Supplied and incomplete, Aug. 31, 1989, Hitach Ltd. Derwent Abstract (008117612) of Japanese Pat. #JP1288321 A, Pub. Nov. 20, 1989, to Matsushita Elec. Ind. Co. (Abstracts of Japan (70 M 1138)) Japanese Abstract #3-101926 A, Pub. Apr. 26, 1991, "Demisting Plastic", to Noguchi. Abstract of Japanese JP6278241 A, Pub. Oct. 4, 1994 "Building Material", Hasegawa et al. Transparent, Photocatalytic TiO2 Film Formed on Glass, Japan Chemical Week, p 4, Dec. 15, 1994. Primary Examiner: Padgett; Marianne Attorney, Agent or Firm: Jones Day Parent Case Text This application is a continuation of application Ser. No. 08/933,886 filed Sep. 19, 1997, now U.S. Pat. No. 6,013,372, which is a continuation-in-part of International application Ser. No. PCT/JP96/00733 filed Mar. 21, 1996 and which designated the United States. Claims What is claimed is: 1. A method of preventing or reducing fogging of a surface of a composite when subjected to humid conditions, comprising: providing a composite with a surface, said composite comprising a substrate and a photocatalytic surface layer, said photocatalytic surface layer comprising a photocatalyst; subjecting the photocatalyst to photoexcitation by exposing the composite to sunlight to render the surface of the composite hydrophilic, wherein, after said photoexcitation, the surface of the composite has a water wettability of less than 10.degree. in terms of the contact angle with water; and subjecting the composite to humidity that is sufficient to induce fogging of said substrate if said photocatalytic surface layer were absent. 2. The method of claim 1, wherein, after said photoexcitation, the surface of the composite has a water wettability of less than 5.degree. in terms of the contact angle with water. 3. The method of claim 1, wherein, after said photoexcitation, the surface of the composite has a water wettability of about 0.degree. in terms of the contact angle with water. 4. The method of claim 1, wherein said photocatalyst is selected from the group consisting of TiO.sub.2, ZnO, SnO.sub.2, SrTiO.sub.3, WO.sub.3, Bi.sub.2 O.sub.3 and Fe.sub.2 O.sub.3. 5. The method of claim 4, wherein said photocatalytic surface layer further comprises a metal selected from the group consisting of Ag, Cu and Zn. 6. The method of claim 4, wherein said photocatalytic surface layer ether comprises a metal selected from the group consisting of Pt, Pd, Rh, Ru, Os and Ir. 7. The method of claim 1, wherein said substrate comprises glass. 8. The method of claim 1, wherein, said substrate comprises glass containing alkaline network modifier ions, and wherein said composite further comprises a film disposed between said substrate and said photocatalytic surface layer, said film preventing ions from diffusing from said substrate into said photocatalytic surface layer. 9. The method of claim 8, wherein said film comprises silica. 10. The method of claim 1, wherein said photocatalytic surface layer further comprises silica or silicone. 11. The method of claim 1, wherein said photocatalytic surface layer consists essentially of said photocatalyst. 12. A method for maintaining a surface of a composite in a clean state when subjected to dirt in air and precipitation, comprising: providing a composite with a surface, said composite comprising a substrate and a photocatalytic surface layer, said photocatalytic surface layer comprising a photocatalyst; subjecting the photocatalyst to photoexcitation by exposing the composite to sunlight to render the surface of the composite hydrophilic, wherein, after said photoexcitation, the surface of the composite has a water wettability of less than about 20.degree. in terms of the contact angle with water; subjecting said composite to dirt in air or precipitation; and washing away the dirt on the surface of the composite by occasional contact with water. 13. The method of claim 12, wherein, after said photoexcitation, the surface of the composite has a water wettability of less than 10.degree. in terms of the contact angle with water. 14. The method of claim 12, wherein, after said photoexcitation, the surface of the composite has a water wettability of less than 5.degree. in terms of the contact angle with water. 15. The method of claim 12, wherein, after said photoexcitation, the surface of the composite has a water wettability of about 0.degree. in terms of the contact angle with water. 16. The method of claim 12, wherein said photocatalyst is selected from the group consisting of TiO.sub.2, ZnO, SnO.sub.2, SrTiO.sub.3, WO.sub.3, Bi2O.sub.3 and Fe.sub.2 O.sub.3. 17. The method of claim 16, wherein said photocatalytic surface layer further comprises a metal selected from group consisting of Ag, Cu and Zn. 18. The method of claim 16, wherein said photocatalytic surface layer further comprises a metal selected from the group consisting of Pt, Pd, Rh, Ru, Os and Ir. 19. The method of claim 12, wherein said substrate comprises glass containing alkaline network modifier ions, and wherein said composite further comprises a film disposed between said substrate and said photocatalytic surface layer, said film preventing ions from diffusing from said substrate and photocatalytic surface layer. 20. The method of claim 19, wherein said film comprises silica. 21. The method of claim 12, wherein said substrate is a tile, a portion of the body of a motor vehicle, an inner panel of a building, or an outer panel of a building. 22. The method of claim 12, wherein said photocatalytic surface layer further comprises silica. 23. The method of claim 12, wherein said photocatalytic surface layer consists essentially of said photocatalyst. Description BACKGROUND OF THE INVENTION 1. Field of the Invention The present invention relates broadly to the art of rendering and maintaining a surface of a substrate highly hydrophilic. More particularly, the present invention relates to the antifogging art wherein the surface of a transparent substrate such as a mirror, lens and sheet glass is made highly hydrophilic to thereby prevent fogging of the substrate or formation of water droplets. This invention is also concerned with the art wherein the surface of a building, windowpane, machinery or article is rendered highly hydrophilic in order to prevent fouling of, to permit self-cleaning of or to facilitate cleaning of the surface. This invention also relates to a hydrophilifiable member having a surface layer which is capable of having an extremely small contact angle with water, a method for rendering the member hydrophilic, a method for forming a hydrophilifiable surface layer, and a coating composition for forming a hydrophilifiable surface layer. 2. Description of the Prior Art It is often experienced that, in the cold seasons, windshields and window-glasses of automobiles and other vehicles, windowpanes of buildings, lenses of eyeglasses, and cover glasses of various instruments are fogged by moisture condensate. Similarly, in a bathroom or lavatory, it is often encountered that mirrors and eyeglass lenses are fogged by steam. Fogging of the surface of an article results from the fact that, when the surface is held at a temperature lower than the dew point of the ambient atmosphere, condensation of moisture in the ambient air takes place to form moisture condensate at the surface. If the condensate particles are sufficiently fine and small so that the diameter thereof is on the order of one half of the wavelength of the visible light, the particles cause scattering of light whereby window-glasses and mirrors become apparently opaque thereby giving rise to a loss of visibility. When condensation of moisture further proceeds so that fine condensate particles are merged together to grow into discrete larger droplets, the refraction of light taking place at the interface between the droplets and the surface and between the droplets and the ambient air causes the surface to be blurred, dimmed, mottled, or clouded. As a result, an image viewed through a transparent article such as sheet glass may become distorted, or the reflected image in a mirror may be disturbed. Similarly, when windshields and window-glasses of vehicles, windowpanes of buildings, rearview mirrors of vehicles, lenses of eyeglasses, or shields of masks or helmets are subjected to rain or water splash so that discrete water droplets are adhered to the surface, their surface is blurred, dimmed, mottled, or clouded, resulting in the loss of visibility. The term "antifogging" as used herein and in the appended claims is intended to mean broadly the art of preventing or minimizing occurrence of optical trouble resulting from fogging, growth of condensate droplets or adherent water droplets mentioned above. The antifogging art can significantly affect safety and efficiency in a variety of setting. For example, the safety of vehicles and traffic can be undermined if the windshields, window-glasses or rearview mirrors of vehicles are fogged or blurred. Fogging of endoscopic lenses and dental mouth mirrors may hinder proper and accurate diagnosis, operation and treatment. If cover glasses of measuring instruments are fogged, a reading of data will become difficult. The windshields of automobiles and other vehicles are normally provided with windshield wipers, defrosting devices and heaters so as to avoid visibility problems, which arise particularly in the cold seasons and under rainy conditions. However, it is not commercially feasible to install this equipment on the side windows of a vehicle, or on the rearview mirrors arranged outside of the vehicle. similarly, it is difficult, if possible at all, to mount such antifogging equipment on windowpanes of buildings, lenses of eyeglasses and endoscopes, dental mouth mirrors, shields of masks and helmets, or cover glasses of measuring instruments. As is well-known, a simple and convenient antifogging method conventionally used in the art is to apply onto a surface an antifogging composition containing either a hydrophilic compound such as polyethylene glycol or a hydrophobic or water-repellent compound such as silicone. However, the disadvantage of this method is that the antifogging coating thus formed is only temporary in nature and is readily removed when rubbed or washed with water so that its effectiveness is prematurely lost. Japanese Utility Model Kokai Publication No. 3-129357 (Mitsubishi Rayon) discloses an antifogging method for a mirror wherein the surface of a substrate is provided with a polymer layer and the layer is subjected to irradiation by ultraviolet light, followed by treatment with an aqueous alkaline solution to thereby form acid radicals at a high density whereby the surface of the polymer layer is rendered hydrophilic. Again, however, it is believed that, according to this method, the hydrophilic property of the surface is degraded as time elapses because of adherent contaminants so that the antifogging function is lost over time. Japanese Utility Model Kokai Publication No. 5-68006(Stanley Electric) discloses an antifogging film made of a graftcopolymer of an acrylic monomer having hydrophilic groups and a monomer having hydrophobic groups. The graftcopolymer is described as having a contact angle with water of about 50.degree.. It is therefore believed that this antifogging film does not exhibit a sufficient antifogging capability. Isao Kaetsu "Antifogging Coating Techniques for Glass", Modern Coating Techniques, pages 237-249, published by Sogo Gijutsu Center (1986), describes various antifogging techniques used in the prior art. The author Mr. Kaetsu nevertheless reports that the prior art antifogging techniques, which consist of rendering a surface hydrophilic, suffer from significant problems which must be overcome in reducing them to practice, and, further reports that the conventional antifogging coating techniques seemingly come up against a barrier. Accordingly, an object of the invention is to provide an antifogging method which is capable of realizing a high degree of visibility in a transparent substrate such as a mirror, lens or glass. Another object of the invention is to provide an antifogging method wherein the surface of a transparent substrate such as a mirror, lens or glass is maintained highly hydrophilic for an extended period of time. Still another object of the invention is to provide an antifogging method wherein the surface of a transparent substrate such as a mirror, lens and glass is almost permanently maintained highly hydrophilic. A further object of the invention is to provide an antifogging coating which has an improved durability and abrasion resistance. Another object of the invention is to provide an antifogging coating which can be readily applied onto a surface requiring antifogging treatment. Yet another object of the invention is to provide an antifogging transparent substrate such as a mirror, lens or glass, as well as a method of making such an antifogging transparent substrate, wherein the substrate surface is maintained highly hydrophilic for an extended period of time to thereby provide a high degree of antifogging property for an extended period. In the fields of architecture and painting, it has been pointed out that growing environmental pollution tends to inadvertently accelerate fouling, contamination or soiling of exterior building materials, including outdoor buildings themselves and the coatings thereon. In this regard, air-borne grime and dust particles are allowed under fair weather conditions to fall and deposit on roofs and outer walls of buildings. When it rains, the deposits are washed away by rainwater and are caused to flow along the outer walls of the buildings. Furthermore, air-borne grime is captured by rain and is carried onto surfaces (such as outer walls) of outdoor structures and buildings, where the grime may flow along or down the surface. For these reasons, contaminant substances are caused to adhere onto the surface along the paths of rainwater. As the surface is dried, a striped pattern of dirt, stain or smudge will appear on the surface. The dirt or stain thus formed on the exterior building materials and exterior coatings consists of contaminant substances which include combustion products such as carbon black, city grime, and inorganic substances such as clay particles. The diversity of the fouling substances is considered to make the antifouling countermeasures complicated (Yoshinori KITSUTAKA, "Accelerated Test Method For soiling on Finishing Materials of External Walls", Bulletin of Japan Architecture Society, vol. 404 (October 1989), pages 15-24). Hitherto, it has been commonly considered in the art that water-repellent paints such as those containing polytetra-fluoroethylene (PTFE) are desirable to prevent fouling or soiling of exterior building materials and the like. Recently, however, it is pointed out that, in order to cope with city grime containing a large amount of oleophilic components, it is rather desirable to render the surface of coatings as hydrophilic as possible ("Highpolymer", vol. 44, May 1995, page 307). Accordingly, it has been proposed in the art to coat a building with a hydrophilic graftcopolymer (Newspaper "Daily Chemical Industry", Jan. 30, 1995). Reportedly, the coating film presents a hydrophilicity of 30-40.degree. in terms of the contact angle with water. However, in view of the fact that inorganic dusts, which may typically be represented by clay minerals, have a contact angle with water ranging from 20 to 50.degree. (so that they have affinity for graftcopolymer having a contact angle with water of 30-40.degree.), it is considered that such inorganic dusts are apt to adhere to the surface of the graftcopolymer coating and, hence, the coating is not able to prevent fouling or contamination by inorganic dusts. Also available in the market are various hydrophilic paints which comprise acrylic resin, acryl-silicone resin, aqueous silicone, block copolymers of silicone resin and acrylic resin, acryl-styrene resin, ethylene oxides of sorbitan fatty acid, esters of sorbitan fatty acid, acetates of urethane, cross-linked urethane of polycarbonatediol and/or polyisocyanate, or cross-linked polymers of alkylester polyacrylate. However, since the contact angle with water of these hydrophilic paints is as large as 50-70.degree., they are not suitable to effectively prevent fouling by city grimes which contain large amount of oleophilic components. Accordingly, a further object of the invention is to provide a method for rendering a surface of a substrate highly hydrophilic and antifouling. Another object of the invention is to provide a method wherein the surface of buildings, window glasses, machinery or articles is rendered highly hydrophilic to thereby prevent fouling of or to permit self-cleaning of or to facilitate cleaning of the surface. Yet another object of the invention is to provide a highly hydrophilic antifouling substrate, as well as a method of making thereof, which is adapted to prevent fouling of or to permit self-cleaning of or to facilitate cleaning of the surface. In certain apparatus, formation of moisture condensate on a surface thereof often hampers operation of the apparatus when condensate has grown into droplets. In heat exchangers, for example, the heat exchanging efficiency would be lowered if condensate particles adhering to radiator fins have grown into large droplets. Accordingly, another object of the invention is to provide a method for preventing adherent moisture condensate from growing into larger water droplets wherein a surface is made highly hydrophilic to thereby permit adherent moisture condensate to spread into a water film. DISCLOSURE OF THE INVENTION The present inventors have found that, upon photoexcitation, a surface of a photocatalyst is rendered highly hydrophilic. Surprisingly, it has been discovered that, upon photoexcitation of photocatalytic titania with ultraviolet light, the surface thereof is rendered highly hydrophilic to the degree that the contact angle with water becomes less than 10.degree., more particularly less than 5.degree., and even reached about 0.degree.. Based on the foregoing findings, the present invention provides, broadly, a method for rendering a surface of a substrate highly hydrophilic, a substrate having a highly hydrophilic surface and a method of making such a substrate. According to the invention, the surface of the substrate is coated with an abrasion-resistant photocatalytic coating comprised of a photocatalytic semiconductor material. Upon irradiation for a sufficient time with a sufficient intensity of a light having a wavelength which has an energy higher than the bandgap energy of the photocatalytic semiconductor, the surface of the photocatalytic coating is rendered highly hydrophilic to exhibit a super-hydrophilicity. The term "super-hydrophilicity" or "super-hydrophilic" as used herein refers to a highly hydrophilic property (i.e., water wettability) of less than about 10.degree. in terms of the contact angle with water. Similarly, the term "superhydrophilification" or "superhydrophilify" refers to rendering a surface highly hydrophilic to the degree that the contact angle with water becomes less than about 10.degree.. It is preferred that a super-hydrophilic drophilic surface have a water wettability of less than about 5.degree.. The process of superhydrophilification of a surface resulting from photoexcitation of a photocatalyst cannot be explained presently with any certainty. seemingly, photocatalytic superhydrophilification is not necessarily identical with photodecomposition of a substance arising from photocatalytic redox process known hitherto in the field of photocatalyst. In this regard, the conventional theory admitted in the art regarding the photocatalytic redox process was that electron-hole pairs are generated upon photoexcitation of the photocatalyst, the electrons thus generated acting to reduce the surface oxygen to produce superoxide ions (O.sub.2.sup.-), the holes acting to oxidize the surface hydroxyl groups to produce hydroxyl radicals (.multidot.OH), these highly active oxygen species (O.sub.2.sup.- and .multidot.OH) then acting to decompose a substance through redox process. However, it seems that the superhydrophilification phenomenon provoked by a photocatalyst is not consistent, in at least two aspects, with the conventional understanding and observation regarding the photocatalytic decomposition process of substances. First, according to a theory widely accepted hitherto, it has been believed that, in a certain photocatalyst such as rutile and tin oxide, the energy level of the conduction band is not high enough to promote the reduction process so that the electrons photoexcited up to the conduction band remain unused and become excessive whereby the electron-hole pairs once generated by photoexcitation undergo recombination without contributing in the redox process. In contrast, the present inventors have observed that the super-hydrophilification process by a photocatalyst takes place even with rutile and tin oxide, as described later. Secondly, the conventional wisdom was that the decomposition of substances due to photocatalytic redox process is not developed unless the thickness of a photocatalytic layer is greater than at least 100 nm. Conversely, the present inventors have found that photocatalytic superhydrophilification occurs even with a photocatalytic coating having a thickness on the order of several nanometers. Accordingly, it is believed (though it cannot be confirmed with certainty) that the superhydrophilification process caused by a photocatalyst is a phenomenon somewhat different from photodecomposition of substances resulting from the photocatalytic redox process. As described later, it has been observed that superhydrophilification of a surface does not occur unless a light having an energy higher than the band gap energy of the photocatalyst is irradiated. It is considered that, presumably, the surface of a photocatalytic coating is rendered superhydrophilic as a result of water being chemisorbed thereon in the form of hydroxyl groups (OH.sup.-) under the photocatalytic action of the photocatalyst. Once the surface of the photocatalytic coating has been made highly hydrophilic upon photoexcitation of the photocatalyst, the hydrophilicity of the surface will be sustained for a certain period of time even if the substrate is placed in the dark. As time elapses, the superhydrophilicity of the surface will be gradually lost because of contaminants adsorbed on the surface hydroxyl groups. However, the superhydrophilicity will be restored when the surface is again subjected to photoexcitation. To initially superhydrophilify the photocatalytic coating, any suitable source of light may be used which has a wavelength of an energy higher than the band gap energy of the photocatalyst. In the case of those photocatalysts such as titania for which the photoexciting wavelength is in the ultraviolet range of the spectrum, the ultraviolet light contained in the sunlight may advantageously be used as the photoexciting light source if the sunlight impinges upon the substrate coated by the photocatalytic coating. When the photocatalyst is to be photoexcited indoors or at night, an artificial light source may be used. In the case where the photocatalytic coating is made of silica blended titania as described later, the surface thereof can readily be rendered hydrophilic even by a weak ultraviolet radiation contained in the light emitted from a fluorescent lamp. After the surface of the photocatalytic coating has once been superhydrophilified, the superhydrophilicity may be maintained or renewed by a relatively weak light. In the case of titania, for example, maintenance and restoration of the superhydrophilicity may be accomplished to a satisfactory degree even by a weak ultraviolet light contained in the light of indoor illumination lamps such as fluorescent lamps. The photocatalytic coating of the present invention exhibits super-hydrophilicity even if the thickness thereof is made extremely small. It presents a sufficient hardness when made, in particular, from a photocatalytic semiconductor material comprising a metal oxide. Therefore, the present photocatalytic coating may have an adequate durability and abrasion resistivity. Superhydrophilification of a surface may be utilized for various applications. In one aspect of the invention, this invention provides an antifogging method for a transparent member, or provides an antifogging transparent member, or provides a method of making an antifogging member. According to the invention, a transparent member is prepared, and the surface of the transparent member is coated with a photocatalytic coating. The transparent member may include a mirror such as a rearview mirror for a vehicle, a bathroom or lavatory mirror, a dental mouth mirror, or a road mirror; a lens such as an eyeglass lens, optical lens, photographic lens, endoscopic lens, or light projecting lens; a prism; a windowpane for a building or control tower; a windowpane for a vehicle such as an automobile, railway vehicle, aircraft, watercraft, submarine, snowmobile, ropeway gondola, pleasure garden gondola and spacecraft; a windshield for a vehicle such as an automobile, railway vehicle, aircraft, watercraft, submarine, snowmobile, motorcycle, ropeway gondola, pleasure garden gondola and spacecraft; a shield for protective or sporting goggles or mask including diving mask; a shield for a helmet; a show window glass for chilled foods; or a cover glass for a measuring instrument. Upon subjecting the transparent member provided with the photocatalytic coating to irradiation by a light to thereby photoexcite the photocatalyst, the surface of the photocatalytic coating will be superhydrophilified. Thereafter, in the event that moisture in the air or steam undergoes condensation, the condensate will be transformed into a relatively uniform film of water without forming discrete water droplets. As a result, the surface will be free from the formation of a light diffusing fog. Similarly, in the event that a windowpane, a rearview mirror of a vehicle, a windshield of a vehicle, eyeglass lenses, a helmet shield, or other substrate is subjected to a rainfall or a splash of water, the water droplets adhering onto the surface will be quickly spread over into a uniform water film thereby preventing formation of discrete water droplets which would otherwise hinder visibility through, or reflection from, the substrate. Accordingly, a high degree of view and visibility is secured so that the safety of vehicle and traffic is secured and the efficiency of various activities improved. In another aspect, this invention provides a method for self-cleaning a surface of a substrate wherein the surface is superhydrophilified and is self-cleaned by rainfall. This invention also provides a self-cleaning substrate and a method of making thereof. The substrate may be any of a variety of articles, including an exterior member, window sash, structural member, or windowpane of a building; an exterior member or coating of a vehicle such as automobile, railway vehicle, aircraft, and watercraft; an exterior member, dust cover or coating of a machine, apparatus or article; and an exterior member or coating of a traffic sign, various display devices, and advertisement towers, that are made, for example, of metal, ceramics, glass, plastics, wood, stone, cement, concrete, a combination thereof, a laminate thereof, or other materials. The surface of the substrate is coated with the photocatalytic coating. Since the building, or machine or article disposed outdoors, is exposed to the sunlight during the daytime, the surface of the photocatalytic coating will be rendered highly hydrophilic. Furthermore, the surface will occasionally be subjected to rainfall. Each time the superhydrophilified surface receives a rainfall, dust and grime and contaminants deposited on the surface of the substrate will be washed away by rain whereby the surface is self-cleaned. As the surface of the photocatalytic coating is rendered highly hydrophilic to the degree that the contact angle with water becomes less than about 10.degree., preferably less than about 5.degree., particularly equal to about 0.degree., not only city grime containing large amounts of oleophilic constituents but also inorganic dusts such as clay minerals will be readily washed away from the surface. In this manner, the surface of the substrate will be self-cleaned and kept clean to a high degree under the action of nature. This will permit, for instance, to eliminate or largely reduce cleaning of windowpanes of towering buildings. In still another aspect, this invention provides an antifouling method for a building, window glass, machine, apparatus, or article wherein the surface thereof is provided with a photocatalytic coating and is rendered highly hydrophilic to prevent fouling. The surface thus superhydrophilified will prevent contaminants from adhering to the surface when rainwater which is laden with contaminants originating from air-borne dust and grime flows down along the surface. Therefore, in combination with the above-mentioned self-cleaning function performed by rainfall, the surface of the building and the like may be maintained in a high degree of cleanliness for an extremely long period of time. In a further aspect of the invention, a photocatalytic coating is provided on a surface of an apparatus or article, such as an exterior or interior member of a building, or a windowpane, household, toilet bowl, bath tub, wash basin, lighting fixture, kitchenware, tableware, sink, cooking range, kitchen hood, or ventilation fan, said apparatus or article being made from metal, ceramics, glass, plastics, wood, stone, cement, concrete, a combination thereof, a laminate thereof, or other materials, and the surface is photoexcited as required. When these articles which are fouled by oil or fat are soaked in, wetted with or rinsed by water, fatty dirt and contaminants will be released from the superhydrophilified surface of the photocatalytic coating and will be readily removed therefrom. Accordingly, for example, a tableware fouled by oil or fat may be cleansed without resort to a detergent. In another aspect, this invention provides a method for preventing growth of condensate droplets adhering to a substrate or for causing adherent water droplets to spread into a uniform water film. To this end, the surface of the substrate is coated with a photocatalytic coating. Once the surface of the substrate has been super-hydrophilified upon photoexcitation of the photocatalytic coating, moisture condensate or water droplets that have come to adhere to the surface will be spread over the surface to form a uniform aqueous film. By applying this method, for example, to radiator fins of a heat exchanger, it is possible to prevent fluid passages for a heat exchange medium from being clogged by condensate; thus the present invention may be used to enhance the heat exchange efficiency. Also, when this method is applied to a mirror, lens, windowpane, windshield, pavement, or other such surface, it is possible to promote drying of the surface after wetting with water. The present inventors have further discovered that hydrophilification of a surface layer made of a photocatalyst results from water molecules being physisorbed onto the surface under the photocatalytic action of the photocatalyst. Based on this discovery, the present invention further provides a method and a composite wherein a substrate is coated with a surface layer comprised of a photocatalyst and wherein upon photoexcitation of the photocatalyst the molecules of water are physisorbed by hydrogen bonding onto the surface layer to thereby form a layer of physisorbed water of a high density. As a layer of physisorbed water is formed on the surface of the photocatalytic layer, the surface is readily hydrophilified to a high degree. Due to the presence of the layer of physisorbed water, the hydrophilicity of the surface is maintained for a long period of time even after photoexcitation is discontinued, thereby minimizing the loss of hydrophilicity over time. Moreover, when the photocatalyst is photoexcited again, the hydrophilicity of the surface is readily recovered within a short period of time of irradiation or with a weak irradiation intensity. These features and advantages of the invention as well as other features and advantages thereof will become apparent from the following description. BRIEF DESCRIPTION OF THE DRAWINGS FIG. 1 shows the energy level of the valance band and the conduction band of various semiconductor photocatalysts usable in the present invention; FIGS. 2A and 2B are schematic cross-sectional views in a microscopically enlarged scale of the photocatalytic coating formed on the surface of a substrate and showing the hydroxyl groups being chemisorbed on the surface upon photoexcitation of the photocatalyst; FIGS. 3-5, 7 and 9 are graphs respectively showing the variation, in response to time, of the contact angle with water of various specimens in the Examples as the specimens are subjected to irradiation of ultraviolet light; FIG. 6 shows Raman spectra of a surface of photocatalytic coating made of silicone; FIGS. 8 and 16 are graphs showing the result of pencil hardness tests; FIG. 10 is a graph showing the relationship between the thickness of the photocatalytic coating and the capability of the coating to decompose methyl mercaptan; FIGS. 11A and 11B are front and side elevational views, respectively, of outdoor accelerated fouling testing equipment; FIGS. 12-15 are graphs showing the contact angle with water versus the molar ratio of silica in silica-blended titania; FIG. 17 is a graph showing to what degree various surfaces having different hydrophilicity are fouled by city grime and sludge; FIGS. 18A-18C are graphs showing the variation, in response to time, of the contact angle with water when ultraviolet light having different wavelengths is irradiated on the surface of the photocatalytic coating; FIGS. 19A and 19B, FIGS. 20A and 20B, FIGS. 21A and 21B, FIGS. 22A and 22B, and FIGS. 23A and 23B, respectively, are graphs showing the infrared absorption spectrum of the surface of the photocatalytic coating; and, FIG. 24 is a schematic cross-sectional view in a microscopically enlarged scale of the surface of the photocatalytic coating and showing molecules of water physisorped onto the surface upon photoexcitation of the photocatalyst. DESCRIPTION OF THE PREFERRED EMBODIMENTS A substrate having a surface requiring super-hydrophilification is prepared and is coated with a photocatalytic coating. In the case where the substrate is made from a heat resistive material such as metal, ceramics and glass, the photocatalytic coating may be fixed on the surface of the substrate by sintering particles of a photocatalyst as described later. Alternatively, a thin film of the amorphous form of a precursor of the photocatalyst may be first formed on the surface of the substrate and the amorphous photocatalyst precursor may then be transformed into photoactive photocatalyst by heating and crystallization. In the case where the substrate is formed of a non heat-resistive material such as plastic or is coated with a paint, the photocatalytic coating may be formed by applying onto the surface a photooxidation-resistant coating composition containing the photocatalyst and by curing the coating composition, as described later. When an antifogging mirror is to be manufactured, a reflective coating may be first formed on the substrate and the photocatalytic coating may then be formed on the front surface of the mirror. Alternatively, the reflective coating may be formed on the substrate subsequent to or during the course of the step of coating of the photocatalyst. Photocatalyst The most preferred example of the photocatalyst usable in the photocatalytic coating according to the invention is titania (TiO.sub.2). Titania is harmless, chemically stable and available at a low cost. Furthermore, titania has a high band gap energy and, hence, requires ultraviolet (UV) light for photoexcitation. This means that absorption of the visible light does not occur during the course of photoexcitation so that the coating is free from the problem of coloring which would otherwise occur due to a complementary color component. Accordingly, titania is particularly suitable to coat on a transparent member such as glass, lens and mirror. Both the anatase and rutile forms of titania may be used. The advantage of the anatase form of titania is that a sol in which extremely fine particles of anatase are dispersed is readily available on the market so that it is easy to make an extremely thin film. on the other hand, the advantage of the rutile form of titania is that it can be sintered at a high temperature so that a coating that has excellent strength and abrasion resistance can be obtained. Although the rutile form of titania is lower in the conduction band level than the anatase form as shown in FIG. 1, it may be used as well for the purpose of photocatalytic superhydrophilification. It is believed that, when a substrate 10 is coated with a photocatalytic coating 12 of titania and the coating is subjected to photoexcitation by UV light, water is chemisorbed on the surface in the form of hydroxyl groups (OH.sup.31) as a result of the photocatalytic action, as shown in FIG. 2A. As a result, the surface becomes superhydrophilic. Other photocatalysts which can be used in the photo-catalytic coating according to the invention may include a metal oxide such as ZnO, SnO.sub.2, SrTiO.sub.3, WO.sub.3, Bi.sub.2 O.sub.3, or Fe2O.sub.3, as shown in FIG. 1. It is believed that, similar to titania, these metal oxides are apt to adsorb the surface hydroxyl groups (OH.sup.-) because the metallic element and oxygen are present at the surface. As shown in FIG. 2B, the photocatalytic coating may be formed by blending particles 14 of photocatalyst in a layer 16 of metal oxide. In particular, the surface can be hydrophilified to a high degree when silica or tin oxide is blended in the photocatalyst as described later. Thickness of Photocatalytic Coating In the case that the substrate is made of a transparent material as in the case of glass, a lens and a mirror, it is preferable that the thickness of the photocatalytic coating is not greater than 0.2 .mu.m. With such a thickness, coloring of the photocatalytic coating due to the interference of light can be avoided. Moreover, the thinner the photocatalytic coating is, the more transparent the substrate can be. In addition, the abrasion resistance of the photocatalytic coating is increased with decreasing thickness. The surface of the photocatalytic coating may be covered further by an abrasion-resistant or corrosion-resistant protective layer or other functional film which is susceptible to hydrophilification. Formation of Photocatalytic Layer by Calcination of Amorphous Titania When the substrate is made of a heat resistive material such as metal, ceramics and glass, one of the preferred methods for forming an abrasion resistant photocatalytic coating which exhibits the superhydrophilicity of such a degree that the contact angle with water becomes as small as 0.degree. is to first form a coating of the amorphous form of titania on the surface of the substrate and to then calcine the substrate to thereby transform by phase transition amorphous titania into crystalline titania (i.e., anatase or rutile). Formation of amorphous titania may be carried out by one of the following methods. (1) Hydrolysis and Dehydration Polymerization of Organic Titanium Compound To an alkoxide of titanium, such as tetraethoxytitanium, tetraisopropoxytitanium, tetra-n-propoxytitanium, tetrabuthoxytitanium, or tetramethoxytitanium, is added a hydrolysis inhibitor such as hydrochloric acid and ethylamine, the mixture being diluted by alcohol such as ethanol or propanol. While subjected to partial or complete hydrolysis, the mixture is applied to the surface of the substrate by spray coating, flow coating, spin coating, dip coating, roll coating or any other suitable coating method, followed by drying at a temperature ranging from the ambient temperature to 200.degree. C. Upon drying, hydrolysis of titanium alkoxide will be completed to result in the formation of titanium hydroxide which then undergoes dehydration polymerization whereby a layer of amorphous titania is formed on the surface of the substrate. In lieu of titanium alkoxide, other organic compounds of titanium such as chelate of titanium or acetate of titanium may be employed. (2) Formation of Amorphous Titania from Inorganic Titanium Compound An acidic aqueous solution of an inorganic compound of titanium such as TiCl.sub.14 or Ti(So.sub.4).sub.2 is applied to the surface of a substrate by spray coating, flow coating, spin coating, dip coating, or roll coating. The substrate is then dried at a temperature of 100-200.degree. C. to subject the inorganic compound of titanium to hydrolysis and dehydration polymerization to form a layer of amorphous titania on the surface of the substrate. Alternatively, amorphous titania may be formed on the surface of the substrate by chemical vapor deposition of TiCl.sub.4. (3) Formation of Amorphous Titania by sputtering Amorphous titania may be deposited on the surface of the substrate by bombarding a target of metallic titanium with an electron beam in an oxidizing atmosphere. (4) Calcination Temperature Calcination of amorphous titania may be carried out at a temperature at least higher than the crystallization temperature of anatase. Upon calcination at a temperature of 400-500.degree. C. or more, amorphous titania may be transformed into the anatase form of titania. Upon calcination at a temperature of 600-700.degree. C. or more, amorphous titania may be transformed into the rutile form of titania. (5) Formation of Diffusion Prevention Layer When the substrate is made of materials such as glass or glazed tile which contains alkaline network-modifier ions (e.g., sodium), it is preferable that an intermediate layer of silica and the like is formed between the substrate and the layer of amorphous titania prior to calcination. This arrangement prevents alkaline network-modifier ions from being diffused from the substrate into the photocatalytic coating during calcination of amorphous titania. As a result, superhydrophilification may be accomplished to the degree that the contact angle with water becomes as small as 0.degree.. Photocatalytic Layer of Silica-Blended Titania Another preferred method of forming an abrasion resistant photocatalytic coating which exhibits the superhydrophilicity of such a degree that the contact angle with water approaches or is equal to 0.degree. is to form on the surface of the substrate a photocatalytic coating comprised of a mixture of titania and silica. The ratio of silica to the sum of titania and silica (by mole percent) may be 5-90%, preferably 10-70%, more preferably 10-50%. The formation of a photocatalytic coating comprised of silica-blended titania may be carried out by any of the following methods. (1) A suspension containing particles of the anatase form or rutile form of titania and particles of silica is applied to the surface of a substrate, followed by sintering at a temperature less than the softening point of the substrate. (2) A mixture of a precursor of amorphous silica (e.g., tetraalkoxysilane such as tetraethoxysilane, tetraisopropoxysilane, tetra-n-propoxysilane, tetrabuthoxysilane, and tetramethoxysilane; silanol formed by hydrolysis of tetraalkoxysilane; or polysiloxane having a mean molecular weight of less than 3000) and a crystalline titania sol is applied to the surface of a substrate and is subjected to hydrolysis where desired to form silanol, followed by heating at a temperature higher than about 100.degree. C. to subject the silanol to dehydration polymerization to thereby form a photocatalytic coating wherein titania particles are bound by amorphous silica. In this regard, if dehydration polymerization of silanol is carried out at a temperature higher than about 200.degree. C., polymerization of silanol is accomplished to a high degree so that the alkali resistance of the photocatalytic coating is enhanced. (3) A suspension comprised of particles of silica dispersed in a solution of a precursor of amorphous titania (e.g., an organic compound of titanium such as alkoxide, chelate or acetate of titanium; or an inorganic compound of titanium such as TiCl.sub.4 and Ti(So.sub.4).sub.2) is applied to the surface of a substrate and then the precursor is subjected to hydrolysis and dehydration polymerization at a temperature ranging from the ambient temperature to 200.degree. C. to thereby form a thin film of amorphous titania wherein particles of silica are dispersed. Then, the thin film is heated at a temperature higher than the crystallization temperature of titania but lower than the softening point of the substrate to thereby transform amorphous titania into crystalline titania by phase transition. (4) Added to a solution of a precursor of amorphous titania (e.g., an organic compound of titanium such as an alkoxide, chelate or acetate of titanium; or an inorganic compound of titanium such as TiCl.sub.4 or Ti(SO.sub.4).sub.2) is a precursor of amorphous silica (e.g., a tetraalkoxysilane such as tetraethoxysilane, tetraisopropoxysilane, tetra-n-propoxy-silane, tetrabuthoxysilane, or tetramethoxysilane; a hydrolyzate thereof, i.e., silanol; or a polysiloxane having a mean molecular weight of less than 3000) and the mixture is applied to the surface of a substrate. Then, these precursors are subjected to hydrolysis and dehydration polymerization to form a thin film made of a mixture of amorphous titania and amorphous silica. Thereafter, the thin film is heated at a temperature higher than the crystallization temperature of titania but lower than the softening point of the substrate to thereby transform amorphous titania into crystalline titania by phase transition. Photocatalytic Layer of Tin Oxide-Blended Titania Still another preferred method of forming an abrasion resistant photocatalytic coating which exhibits the superhydrophilicity of such a degree that the contact angle with water is equal to 0.degree. is to form on the surface of a substrate a photocatalytic coating comprised of a mixture of titania and tin oxide. The ratio of tin oxide to the sum of titania and tin oxide may be 1-95% by weight, preferably 1-50% by weight. Formation of a photocatalytic coating comprised of tin oxide-blended titania may be carried out by any of the following methods. (1) A suspension containing particles of the anatase form or rutile form of titania and particles of tin oxide is applied to the surface of a substrate, followed by sintering at a temperature less than the softening point of the substrate. (2) A suspension comprised of particles of tin oxide dispersed in a solution of a precursor of amorphous titania (e.g., an organic compound of titanium such as alkoxide, chelate or acetate of titanium; or an inorganic compound of titanium such as TiCl.sub.4 or Ti(SO.sub.4).sub.2) is applied to the surface of a substrate and then the precursor is subjected to hydrolysis and dehydration polymerization at a temperature ranging from the ambient temperature to 200.degree. C. to thereby form a thin film of amorphous titania wherein particles of tin oxide are dispersed. Then, the thin film is heated at a temperature higher than the crystallization temperature of titania but lower than the softening point of the substrate to thereby transform amorphous titania into crystalline titania by phase transition. Silicone Paint Containing Photocatalyst A further preferred method of forming a photocatalytic coating which exhibits the superhydrophilicity of such a degree that the contact angle with water is equal to 0.degree. is to use a coating composition wherein particles of a photocatalyst are dispersed in a film forming element of uncured or partially cured silicone (organopolysiloxane) or a precursor thereof. The coating composition is applied on the surface of a substrate and the film forming element is then subjected to curing. Upon photoexcitation of the photocatalyst, the organic groups bonded to the silicon atoms of the silicone molecules are substituted with hydroxyl groups under the photocatalytic action of the photocatalyst, as described later with reference to Examples 13 and 14, whereby the surface of the photocatalytic coating is superhydrophilified. This method provides several advantages. Since the photocatalyst-containing silicone paint can be cured at ambient temperature or at a relatively low temperature, this method may be applied to a substrate formed of a non-heat-resistant material such as plastics. The coating composition containing the photocatalyst may be applied whenever desired by way of brush painting, spray coating, roll coating and the like on any existing substrate requiring superhydrophilification of the surface. Superhydrophilification by photoexcitation of the photocatalyst may be readily carried out even by the sunlight as a light source. Furthermore, in the event that the coating film is formed on a plastically deformable substrate such as a steel sheet, it is possible to readily subject the steel sheet to plastic working as desired after curing of the coating film and prior to photoexcitation. Prior to photoexcitation, the organic groups are bonded to the silicon atoms of the silicone molecules so that the coating film has an adequate flexibility. Accordingly, the steel sheet may be readily deformed without damaging the coating film. After plastic deformation, the photocatalyst may be subjected to photoexcitation whereupon the organic groups bonded to the silicon atoms of the silicone molecules will be substituted with hydroxyl groups under the action of photocatalyst to thereby render the surface of the coating film superhydrophilic. It is believed that the photocatalyst-containing silicone paint has a sufficient resistance to photooxidation action of the photocatalyst because it is composed of the siloxane bond. Another advantage of the photocatalytic coating made of photocatalyst-containing silicone paint is that, once the surface has been rendered superhydrophilic, the super-hydrophilicity is maintained for a long period of time even if the coating is kept in the dark and a further advantage is that the superhydrophilicity can be restored even by the light of an indoor illumination lamp such as fluorescent lamp. Examples of the film forming element usable in the invention include methyltrichlorosilane, methyltribromosilane, methyltrimethoxysilane, methyltriethoxysilane, methyltriisopropoxysilane,methyltri-t-buthoxysilane; ethyltrichlorosilane, ethyltribromosilane, ethyltrimethoxysilane, ethyltriethoxysilane, ethyltriisopropoxysilane, ethyltri-t-buthoxysilane; n-propyltrichlorosilane, n-propyltribromosilane, n-propyltrimethoxysilane, n-propyltriethoxysilane, n-propyltriisopropoxysilane, n-propyltri-t-buthoxysilane; n-hexyltrichlorosilane, n-hexyltribromosilane, n-hexyltrimethoxysilane, n-hexyltriethoxysilane, n-hexyltriisopropoxysilane, n-hexyltri-t-buthoxysilane; n-decyltrichlorosilane, n-decyltribromosilane, n-decyltrimethoxysilane, n-decyltriethoxysilane, n-decyltriisopropoxysilane, n-decyltri-t-buthoxysilane; n-octadecyltrichlorosilane, n-octadecyltribromosilane, n-octadecyltrimethoxysilane, n-octadecyltriethoxysilane, n-octadecyltriisopropoxysilane, n-octadecyltri-t-buthoxysilane; phenyltrichlorosilane, phenyltribromosilane, phenyltrimethoxysilane, phenyltriethoxysilane, phenyltriisopropoxysilane, phenyltri-t-buthoxysilane; tetrachlorosilane, tetrabromosilane, tetramethoxysilane, tetraethoxysilane, tetrabuthoxysilane, dimethoxydiethoxysilane; dimethyldichlorosilane, dimethyldibromosilane, dimethyldimethoxysilane, dimethyldiethoxysilane; diphenyldichlorosilane, diphenyldibromosilane, diphenyldimethoxysilane, diphenyldiethoxysilane; phenylmethyldichlorosilane, phenylmethyldibromosilane, phenylmethyldimethoxysilane, phenylmethyldiethoxysilane; trichlorohydrosilane, tribromohydrosilane, trimethoxyhydrosilane, triethoxyhydrosilane, triisopropoxyhydrosilane, tri-t-buthoxyhydrosilane; vinyltrichlorosilane, vinyltribromosilane, vinyltrimethoxysilane, vinyltriethoxysilane, vinyltriisopropoxysilane, vinyltri-t-buthoxysilane; trifluoropropyltrichlorosilane, trifluoropropyltribromosilane, trifluoropropyltrimethoxysilane, trifluoropropyltriethoxl silane, trifluoropropyltriisopropoxysilane, trifluoropropyltri-t-buthoxysilane; gamma-glycidoxypropylmethyldimethoxysilane, gamma-glycidoxypropylmethyldiethoxysilane, gamma-glycidoxypropyltrimethoxysilane gamma-glycidoxypropyltriethoxysilane, gamma-glycidoxypropyltriisopropoxysilane, gamma-glycidoxypropyltri-t-buthoxysilane; gamma-methacryloxypropylmethyl dimethoxysilane, gamma-methacryloxypropylmethyldiethoxysilane, gamma-methacryloxypropyltrimethoxysilane, gamma-methacryloxypropyltriethoxysilane gamma-methacryloxypropyltriisopropoxy silane, gamma-methacryloxypropyltri-t-buthoxysilane ;gamma-aminopropylmethyldimethox ysilane, gamma-aminopropylmethyldiethoxysilane, gamma-aminopropyltrimethoxysilane,gamma-aminopropylmethyldimethoxysilane, gamma-aminopropyltriisopropoxysilane, gamma-aminopropyltri-t-buthoxysilane; gamma-mercaptopropylmethyldimethoxysilane, gamma-mercaptopropylmethyldiethoxysilane, gamma-mercaptopropyltrimethoxysilane, gamma-mercaptopropyltriethoxysilane, gamma-mercaptopropyltriisopropoxysilane, gamma-mercaptopropyltri-t-buthoxysilane; .beta.-(3,4-epoxycyclohexyl)ethyltrimetho xysilane, .beta.-(3,4-epoxycyclohexyl)ethyltriethoxysilane; partial hydrolizates of any of the foregoing; and mixtures of any of the foregoing. To ensure that the silicone coating exhibits a satisfactory hardness and smoothness, it is preferable that the coating contains (by mole percent) more than 10% of a three-dimensionally cross-linking siloxane. In addition, to provide an adequate flexibility of the coating film yet assuring a satisfactory hardness and smoothness, it is preferred that the coating contains less than 60% (by mole percent) of a two-dimensionally cross-linking siloxane. Furthermore, to enhance the speed that the organic groups bonded to the silicon atoms of the silicone molecules are substituted with hydroxyl groups upon photoexcitation, it is desirable to use a silicone wherein the organic groups bonded to the silicon atoms of the silicone molecules are n-propyl or phenyl groups. In place of silicone having siloxane bonds, an organopolysilazane having silazane bonds may be used. Addition of Antibacterial Enhancer The photocatalytic coating may be doped with a metal such as Ag, Cu and Zn. Doping of the photocatalyst with a metal such as Ag, Cu or Zn may be carried out by adding a soluble salt of such metal to a suspension containing particles of the photocatalyst, the resultant solution being used to form the photocatalytic coating. Alternatively, after forming the photocatalytic coating, a soluble salt of such metal may be applied thereon and may be subjected to irradiation of light to deposit the metal by photoreduction. The photocatalytic coating doped with a metal such as Ag, Cu or Zn is capable of killing bacteria adhered to the surface. Moreover, such photocatalytic coating inhibits growth of microorganisms such as mold, algae and moss. As a result, the surface of a building, machine, apparatus, household, article and the like can be maintained clean for a long period. Addition of Photoactivity Enhancer The photocatalytic coating may additionally be doped with a metal of the platinum group such as Pt, Pd, Rh, Ru, Os or Ir. These metals may be similarly doped into the photocatalyst by photoreduction deposition or by addition of a soluble salt. A photocatalyst doped with a metal of the platinum group develops an enhanced photocatalytic redox activity so that decomposition of contaminants adhering on the surface will be promoted. Photoexcitation and UV Irradiation For antifogging purposes (e.g., with respect to a transparent member such as glass, a lens or a mirror), it is preferable that the photocatalytic coating be formed from a photocatalyst such as titania that has a high band gap energy and can be photoexcited only by UV light. In such event, the photocatalytic coating does not absorb visible light so that glass, a lens or a mirror, or other such transparent member, would not be colored by a complementary color component. The anatase form of titania may be photoexcited by a UV light having a wavelength less than 387 nm, with the rutile form of titania by a UV light having a wavelength less than 413 nm, with tin oxide by a UV light having a wavelength less than 344 nm, with zinc oxide by a UV light having a wavelength less than 387 nm. As a source of UV light, a fluorescent lamp, incandescent lamp, metal halide lamp, mercury lamp or other type of indoor illumination lamp may be used. As the antifogging glass, lens or mirror, or other transparent member, is exposed to UV light, the surface thereof will be superhydrophilified by photoexcitation of the photocatalyst. In a situation where the photocatalytic coating is exposed to sunlight as in the case of a rearview mirror of a vehicle, the photocatalyst will advantageously be photoexcited spontaneously by the UV light contained in the sunlight. Photoexcitation may be carried out, or caused to be carried out, until the contact angle, with water, of the surface becomes less than about 10.degree., preferably less than about 5.degree., particularly equal to about 0.degree.. Generally, by photoexciting at a UV intensity of 0.001 mW/cm.sup.2, the photocatalytic coating will be superhydrophilified within several days to the degree that the contact angle with water becomes about 0.degree.. Since the intensity of the UV light contained in the sunlight impinging upon the earth's surface is about 0.1-1 mW/cm.sup.2, the surface will be superhydrophilified in a shorter time when exposed to the sunlight. In the case that the surface of the substrate is to be self-cleaned by rainfall or to be prevented from adhesion of contaminants, the photocatalytic coating may be formed of a photocatalyst which can be photoexcited by UV light or visible light. If the articles covered by the photocatalytic coating are disposed outdoors, they will ordinarily be subjected to irradiation of the sunlight and to rainfall. When the photocatalytic coating is made of titania-containing silicone, it is preferable to photoexcite the photocatalyst at such an intensity to ensure that a sufficient amount of the surface organic groups bonded to the silicon atoms of the silicone molecules are substituted with hydroxyl groups. The most convenient method therefor is to use the sunlight. Once the surface has been made highly hydrophilic, the hydrophilicity is sustained even during the night. Upon exposure again to the sunlight, the hydrophilicity will be restored and maintained. It is preferable that the photocatalytic coating is superhydrophilified in advance before the substrate coated by the photocatalytic coating according to the invention is offered for use to the user. EXAMPLES The following Examples illustrate the industrial applicability of the invention from various aspects. Example 1 Antifogging Mirror--Antifogging Photocatalytic Coating with Interleaved Silica Layer 6 parts by weight of tetraethoxysilane Si(OC.sub.2 H.sub.5).sub.4 (Wako JunYaku, Osaka), 6 parts by weight of pure water, and 2 parts by weight of 36% hydrochloric acid as a hydrolysis inhibitor were added to 86 parts by weight of ethanol as a solvent and the mixture was stirred to obtain a silica coating solution. The solution was allowed to cool for about 1 hour since the solution evolved heat upon mixing. The solution was then applied on the surface of a soda-lime glass plate of 10 cm square in size by the flow coating method and was dried at a temperature of 80.degree. C. As drying proceeds, tetraethoxysilane was hydrolyzed to first form silanol Si(OH).sub.4 which then underwent dehydration polymerization to form a thin film of amorphous silica on the surface of the glass plate. Then a titania coating solution was prepared by adding 0.1 parts by weight of 36% hydrochloric acid as a hydrolysis inhibitor to a mixture of 1 part by weight of tetraethoxy-titanium (Ti(OC.sub.2 H.sub.5).sub.4)(Merck) and 9 parts by weight of ethanol, and the solution was applied to the surface of the above-mentioned glass plate by the flow coating method in dry air. The amount of coating was 45 .mu.g/cm.sup.2 in terms of titania. As the speed of hydrolysis of tetraethoxytitanium was so high, hydrolysis of tetraethoxytitanium partially commenced during the course of coating so that formation of titanium hydroxide Ti(OH).sub.4 started. Then the glass plate was held at a temperature of about 150.degree. C. for 1-10 minutes to permit completion of the hydrolysis of tetraethoxy-titanium and to subject the resultant titanium hydroxide to dehydration polymerization whereby amorphous titania was formed. In this manner, a glass plate was obtained having a coating of amorphous titania overlying the coating of amorphous silica. This specimen was then fired or calcined at a temperature of 500.degree. C. in order to transform amorphous titania into the anatase form of titania. It is considered that, due to the presence of the coating of amorphous silica underlying the coating of amorphous titania, alkaline network-modifier ions (such as sodium ions present in the glass plate) were prevented from diffusing from the glass substrate into the titania coating during calcination. Then a reflective coating of aluminum was formed by vacuum evaporation deposition on the back of the glass plate to prepare a mirror to thereby obtain #1 specimen. After the #1 specimen was kept in the dark for several days, a UV light was irradiated on the surface of the specimen for about one hour at a UV intensity of 0.5 mW/cm.sup.2 (the intensity of UV light having an energy higher than the band gap energy of the anatase form of titania, i.e., the intensity of UV light having a wavelength shorter than 387 nm) by using a 20 W blue-light-black (BLB) fluorescent lamp (Sankyo Electric, FL20BLB) to obtain #2 specimen. For the purposes of comparison, a reflective coating of aluminum was formed by vacuum evaporation deposition on the back of a glass plate provided neither with silica nor titania coating, the product being placed in the dark for several days to obtain #3 specimen. The contact angle, with water, of the #2 and #3 specimens was measured by a contact angle meter (Kyowa Kaimen Kagaku K.K. of Asaka, Saitama, Model CA-X150). The resolving power at the small angle side of this contact angle meter was 1.degree.. The contact angle was measured 30 seconds after a water droplet was dripped from a micro-syringe onto the surface of the respective specimens. In the #2 specimen, the reading of the contact angle meter was Of so that the surface exhibited superhydrophilicity. In contrast, the contact angle with water of the #3 specimen was 30-40.degree.. Then the #2 and #3 specimens were tested for antifogging capability, as well as to see how adherent water droplets would spread over the surface. Assessment of the antifogging capability was done by filling a 500 ml beaker with 300 ml of hot water at about 80.degree. C., and thereafter placing each specimen on the beaker for about 10 seconds with the front surface of the mirror directed downwards, and then inspecting immediately thereafter the presence or absence of a fog on the surface of the specimen and inspecting how the face of the tester reflected. With the #3 specimen, the surface of the mirror was fogged by steam so that the image of the observer's face was not reflected well. However, with the #2 specimen, no fogging was observed at all and the face of the tester was clearly reflected. Assessment of the spreading of adherent water droplets was carried out by dripping several water droplets from a pipette onto the mirror surface of each specimen inclined at an angle of 45.degree., rotating the mirror into a vertical position, and thereafter inspecting how the droplets adhered and how the face of the observer reflected. With the #3 specimen, dispersed discrete water droplets which were obstructive to the eye adhered on the mirror surface. As a result, the reflected image was disturbed by the refraction of light due to adherent droplets so that it was difficult to observe the reflected image with clarity. In contrast, with the #2 specimen, water droplets adhered onto the mirror surface were allowed to spread over the surface to form a relatively uniform water film without forming discrete water droplets. Although a slight distortion of the reflected image due to the presence of the water film was observed, it was possible to recognize the reflected image of the tester's face with a sufficient clarity. Example 2 Antifogging Mirror--Photocatalytic Coating Comprising Silica-Blended Titania A thin film of amorphous silica was formed on the surface of a mirror (made by Nihon Flat Glass, MFL3) in a manner similar to Example 1. Then a coating solution was prepared by admixing 0.69 g of tetraethoxysilane (Wako JunYaku), 1.07 g of a sol of the anatase form of titania (Nissan Chemical Ind., TA-15, mean particle size of 0.01 .mu.m), 29.88 g of ethanol, and 0.36 g of pure water. The coating solution was applied on the surface of the mirror by spray coating process. The mirror was held at a temperature of about 150.degree. C. for about 20 minutes to subject tetraethoxysilane to hydrolysis and dehydration polymerization to thereby form on the mirror surface a coating wherein particles of the anatase form of titania were bound by a binder of amorphous silica. The ratio by weight of titania to silica was 1. After the mirror was kept in the dark for several days, a UV light was irradiated by the BLB fluorescent lamp for about one hour, at a UV intensity of 0.5 mW/cm.sub.2 to obtain #1 specimen. The contact angle with water at the surface of the mirror was measured by the same contact angle meter as used in Example 1 and the reading of the contact angle meter was 0.degree.. Then, in the manner similar to Example 1, the antifogging capability and the spreading of adherent water droplets were assessed with respect to the coated #1 specimen, as well as to an "MFL3" mirror specimen not provided with a photocatalytic coating. In the test for antifogging property, with the coated #1 specimen, no fog was observed at all and the tester's face was clearly reflected, in contrast to the uncoated "MFL3" mirror wherein a fog was observed on the surface of the mirror so that the image of the tester's face was not clearly reflected. In the inspection for spreading of adherent water droplets, with the uncoated "MFL3" mirror, water droplets remained as droplets on the surface, causing refraction of light and thereby disturbing the reflected image, so that it was difficult to clearly observe the reflected image. With the coated #1 specimen, in contrast, water droplets on the mirror were spread over the surface to form a relatively uniform water film and, although a slight distortion was observed in the reflected image due to the presence of the water film, it was possible to recognize the reflected image of the tester's face with sufficient clarity. Example 3 Antifogging Eyeclass Lens First, a thin film of amorphous silica was formed in a manner similar to Example 1 on both sides of an eyeglass lens commercially available on the market. Then, the coating solution similar to that of Example 2 was spray coated on both sides of the lens and the lens was held at a temperature of about 150.degree. C. for about 20 minutes to subject tetraethoxysilane to hydrolysis and dehydration polymerization to thereby form on each side of the lens a coating wherein particles of the anatase form of titania were bound by a binder of amorphous silica. After the lens was kept in the dark for several days, it was irradiated with UV light from a BLB fluorescent lamp for about one hour at a UV intensity of 0.5 mW/cm.sup.2. When the contact angle with water at the surface of the lens was measured by the same contact angle meter as used in Example 1, the reading of the contact angle meter was 0.degree.. This lens was mounted to the right-hand side of a pair of eyeglasses, with an ordinary lens being mounted for the purposes of comparison to the left-hand side. When, several hours later, the tester wore the glasses and took a bath for about 5 minutes, the ordinary lens on the left was fogged with steam so that the eyesight was lost. However, formation of fog was not observed at all on the right-hand lens coated with the photocatalytic coating that had been subjected to UV irradiation. As the tester then intentionally directed a shower on the glasses, obstructive water droplets adhered on the left-hand ordinary lens so that a view was interrupted. However, water droplets on the right-hand lens promptly spread into a water film so that a sufficient view, with adequate clarity, was secured. Example 4 Antifogging Glass--7 nm Thick Titania Coating A solution containing a chelate of titanium was applied to the surface of a soda-lime glass plate (10 cm square in size) and the titanium chelate was subjected to hydrolysis and dehydration polymerization to form amorphous titania on the surface of the glass plate. The plate was then calcined at a temperature of 500.degree. C. to form a surface layer of crystals of the anatase form of titania. The thickness of the surface layer was 7 nm. The surface of the thus obtained specimen was first subjected to irradiation with UV light for about one hour, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. The contact angle with water of the surface of this specimen was measured by a contact angle meter (made by ERMA, Model G-I-1000, the resolving power at the small angle side being 3.degree.), and the reading of the contact angle meter was less than 3.degree.. Then, while irradiating with UV light at a UV intensity of 0.01 mW/cm.sup.2 by using a 20 W white fluorescent lamp (Toshiba, FL20SW), the variation, in response to time, of the contact angle was measured. The results are plotted in the graph of FIG. 3. It will be noted from the graph that the surface of the specimen was maintained highly hydrophilic even by a weak UV light emitted from the white fluorescent lamp. This Example illustrates that the surface of the photocatalytic titania coating can be maintained highly hydrophilic even though the thickness thereof is made as extremely small as 7 nm. This is very important in preserving the transparency of a substrate such as a windowpane. Example 5 Antifogging Glass--20 nm Thick Titania coating A surface layer of anatase-form titania crystals was formed on the surface of a soda-lime glass plate in a manner similar to Example 4. The thickness of the surface layer was 20 nm. Similar to Example 4, the surface of the thus obtained specimen was first subjected to irradiation with UV light for about one hour, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. Then, the variation in response to time of the contact angle was measured while subjecting the specimen to irradiation with UV light at a UV intensity of 0.01 mW/cm.sup.2, by using a white fluorescent lamp. The results are shown in the graph of FIG. 4. In this Example, too, the surface of the specimen was maintained highly hydrophilic by a weak UV light emitted from a white fluorescent lamp. Example 6 Antifogging Glass--Effect of Calcination Temperature of Amorphous Titania In a manner similar to Example 1, a thin film of amorphous silica was first formed on the surface of soda-lime glass plates (each 10 cm square in size) and then a thin film of amorphous titania was coated thereon to obtain a plurality of specimens. These glass plates were then calcined at temperatures of 450.degree. C., 475.degree. C., 500.degree. C., and 525.degree. C., respectively. Upon inspection by the powder X-ray diffraction method, the presence of crystalline titania of the anatase form was detected in the specimens calcined at 475.degree. C., 500.degree. C., and 525.degree. C. so that transformation of amorphous titania into the anatase form crystalline titania was confirmed in these specimens. However, in the specimen calcined at 450.degree. C., the anatase form of titania was not detected. The surface of the thus obtained specimens was first subjected to irradiation with UV light for about three hours, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. Then, the variation in response to time of the contact angle was measured by the contact angle meter (CA-X150) while subjecting the specimen to irradiation with UV light, at a UV intensity of 0.02 mW/cm.sup.2, by using a white fluorescent lamp. The results are shown in Table 1. TABLE 1 Contact Angle (.degree.) Calcination immed. aft Temp (.degree. C.) BLB irradn 450 10 475 0 500 0 525 0 3 days later 13 0 0 0 9 days later 15 0 0 0 14 days later 23 0 0 0 As will be apparent from Table 1, it was found that, in the specimens which were calcined at temperatures of 475.degree. C., 500.degree. C., and 525.degree. C., respectively, and in which the formation of anatase crystals were confirmed, the contact angle was maintained at 0.degree. and the surface of the glass plate was maintained superhydrophilic as long as irradiation with UV light by a white fluorescent lamp was continued. In contrast, it was observed that the coating of amorphous titania of the specimen calcined at 450.degree. C. did not exhibit photocatalytic activity so that the contact angle increased as time elapsed. When a blow of breath was blown upon the specimens calcined at temperatures of 475.degree. C., 500.degree. C., and 525.degree. C., respectively, no formation of fog was observed on the specimen surfaces. Example 7 Antifogging Glass--Effect of Alkaline Network Modifier Ion Diffusion A titania coating solution similar to Example 1 was prepared and was applied by the flow coating method on the surface of a soda-lime glass plate (10 cm square in size). Similar to Example 1, the amount of coating was 45 .mu.g/cm.sup.2 in terms of titania. The glass plate was similarly held at a temperature of about 150.degree. C. for 1-10 minutes to form amorphous titania on the surface of the glass plate. The specimen was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form of titania. After keeping the specimen in the dark for several days, UV light was irradiated on the surface of the specimen for about one hour, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. Thereafter, the contact angle with water was measured by the contact angle meter (CA-X150), which indicated a contact angle of 3.degree.. It is considered that the reason why the contact angle for this specimen was not reduced to 0.degree. is that because, contrary to Example 1, the specimen of this Example was not provided with a silica layer interleaved between the glass substrate and the titania layer. Thus, the alkaline network-modifier ions (such as sodium ions) were allowed to diffuse from the glass substrate into the titania coating during calcination at 500.degree. C., whereby the photocatalytic activity of titania was hindered. It is therefore believed that, in order to realize the superhydrophilicity of such a degree that the contact angle with water is equal to 0.degree., it is preferable to provide an intermediate layer of silica as in Example 1. Example 8 Antifogging Glass--Formation of Amorphous Titania By Sputtering A film of metallic titanium was deposited by sputtering on. the surface of a soda-lime glass plate (10 cm square in size). The glass plate was then calcined at a temperature of 500.degree. C. Upon inspection by the powder X-ray diffraction method, formation of the anatase form of titania was observed on the surface of the glass plate. Metallic titanium was apparently oxidized into the anatase form by calcination. Soon after calcination, the surface of the specimen was subjected to irradiation with UV light, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. The contact angle with water was then measured by the contact angle meter (CA-X150) to monitor the variation in response to time of the contact angle, while irradiation continued. The results are shown in the graph of FIG. 5. As will be apparent from the graph, the contact angle with water was kept less than 3.degree.. This experiment illustrates that, even in the case where the photocatalytic coating is formed by sputtering, the surface of a glass plate is maintained highly hydrophilic upon UV irradiation. Example 9 Antifogging Glass--UV Intensity of 800 Lux A thin film of amorphous silica was formed on the surface of a 10 cm-square soda-lime glass plate in a manner similar to Example 1. Then the coating solution of Example 2 was applied by spray coating on the surface of the glass plate. The glass plate was then held at a temperature of about 150.degree. C. for about 20 minutes whereby a coating in which particles of the anatase form of titania were bound by a binder of amorphous silica was formed on the surface of the glass plate. The ratio by weight of titania to silica was 1. After being kept in the dark for several days, the glass plate was subjected to irradiation with UV light for about one hour, at a UV intensity of 0.5 mW/cm.sup.2, by a BLB fluorescent lamp. After UV irradiation, the contact angle with water of the surface of the glass plate was measured by the contact angle meter (CA-X150) and it was found that the contact angle was 0.degree.. Thereafter, the specimen was subjected to irradiation with UV light for 4 days, at a UV intensity of 0.004 mW/cm.sup.2 (800 lux), by using a white fluorescent lamp. While the specimen was under UV irradiation, the contact angle at the surface thereof was maintained less than 2.degree.. When 4 days later a blow of breath was blown upon the specimen, formation of fog was not observed. In this way, it was confirmed that, even with a weak UV light such as is available for indoor illumination achieved, for example, by a white fluorescent lamp, the surface of the glass plate was maintained highly hydrophilic and fogging of the glass plate was prevented. Example 10 Antifogging Glass--Effect of Silica-to-Titania Blending Ratio Next, tetraethoxysilane (Wako JunYaku), a sol of the anatase form of titania (Nissan Chemical Ind., TA-15), ethanol, and pure water were admixed in varying rate to prepare four kinds of coating solutions having different tetraethoxysilane-to-titania sol blending ratios. The ratios of tetraethoxysilane to titania sol were selected so that, after tetraethoxysilane was converted into amorphous silica, the ratio of silica to the sum of silica plus titania was equal, by mole percent, to 10%, 30%, 50%, and 70%, respectively. Each of the coating solutions was applied by spray coating on the surface of a 10 cm-square soda-lime glass plate which was then held at a temperature of about 150.degree. C for about 20 minutes to subject tetraethoxysilane to hydrolysis and dehydration polymerization. Thus, a coating in which particles of the anatase form of titania were bound by a binder of amorphous silica was formed on the surface of the glass plate. After being kept in the dark for a week, the specimens were subjected to irradiation with UV light for about one hour, at a UV intensity of 0.3 mW/cm.sup.2, by a BLB fluorescent lamp. After UV irradiation, the contact angle with water was measured for the surface of the respective specimens using the contact angle meter (CA-X150). The contact angle was 0.degree. throughout all the specimens. Thereafter, two specimens with coatings having 30% by mol and 50% by mol of silica, respectively, were subjected to irradiation with UV light for 3 days, at a UV intensity of 0.004 mW/cm.sup.2, by using a white fluorescent lamp. While the specimens were under irradiation, the contact angle at the surface thereof was maintained less than 3.degree.. Example 11 Antifogging Glass--Rutile Form Photocatalytic Coating A titania coating solution was prepared by adding 0.1 part by weight of 36% hydrochloric acid as a hydrolysis inhibitor to a mixture of 1 part by weight of tetraethoxytitanium (Ti(OC.sub.2 H.sub.5).sub.4) (Merck) and 9 parts by weight of ethanol. The solution was then applied to the surface of a plurality of quartz glass plates (10 cm square in size) by the flow coating method in dry air. The amount of coating was 45 .mu.g/cm.sup.2 in terms of titania. The glass plates were then held at a temperature of about 150.degree. C. for 1-10 minutes to subject tetraethoxytitanium to hydrolysis and dehydration polymerization whereby a coating of amorphous titania was formed on the surface of each glass plate. These specimens were then calcined at temperatures of 650.degree. C. and 800.degree. C., respectively, to subject amorphous titania to crystallization. Upon inspection by the powder X-ray diffraction method, it was found that the crystal form of the specimen calcined at 650.degree. C. was of the anatase form while the crystal form of the specimen calcined at 800.degree. C. was of the rutile form. After keeping the thus obtained specimens in the dark for a week, they were subjected to irradiation with UV light for 2 days, at a UV intensity of 0.3 mW/cm.sup.2, by a BLB fluorescent lamp. After UV irradiation, the contact angle was measured. The contact angle with water of the surface was 0.degree. throughout all the specimens. It will be understood from the foregoing that a surface can be maintained highly hydrophilic not only in the case that the photocatalyst is the anatase form of titania but also in the case that the photocatalyst is the rutile form. For this reason, it seems that the phenomenon of photocatalytic superhydrophilification is not altogether the same as the photocatalytic redox reaction. Example 12 Antifogging Glass--Transmittance Test In a manner similar to Example 1, a thin film of amorphous silica was first formed on the surface of a soda-lime glass plate (10 cm square in size) and then a thin film of amorphous titania was coated thereon. The glass plate was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form of titania. The specimen thus obtained was kept in the dark for several days. Then the specimen was placed in a desiccator (24.degree. C. in temperature and 45-50% in humidity) housing a BLB fluorescent lamp and was subjected to irradiation with UV light for one day, at a UV intensity of 0.5 mW/cm.sup.2, to obtain #1 specimen. The contact angle with water of the #1 specimen as measured was 0.degree.. Then the #1 specimen was taken out of the desiccator and was promptly positioned above a warm bath held at 60.degree. C. and transmittance was measured 15 seconds later. The transmittance as measured was divided by the initial transmittance to calculate a change in transmittance caused by any fog formed through condensation of steam. In a manner similar to Example 7, the surface of a glass plate was coated by the anatase form of titania to obtain #2 specimen. The #2 specimen was placed in the desiccator and was subjected to irradiation with UV light, at a UV intensity of 0.5 mW/cm.sup.2, until the contact angle with water became equal to 3.degree.. The #2 specimen was then placed in a dark place. The #2 specimen was taken out of the dark place at different time points and each time the contact angle with water was measured. In addition, at each point, the #2 specimen was placed in the desiccator (24.degree. C. in temperature and 45-50% in humidity) until the temperature was equalized whereupon, in a manner similar to the #1 specimen, the #2 specimen was promptly placed above a warm bath held at 60.degree. C. and the transmittance was measured 15 seconds later to derive a change in transmittance caused by a fog formed by condensation of steam. For purposes of comparison, the contact angle with water was also measured with respect to commercially marketed flat glass, acrylic resin plate, polyvinylchloride (PCV) plate and polycarbonate (PC) plate, respectively. Thereafter, each of these materials was placed in a desiccator, held under the same condition, to equalize the temperature and was then promptly placed above a warm bath held at 60.degree. C., the transmittance being similarly measured 15 seconds later whereby a change in transmittance caused by a fog formed by condensation of steam was calculated. The results are shown in Table 2. TABLE 2 Specimen #1 #2 (3 hrs later) #2 (6 hrs later) Contact Angle with Change in Water (.degree.) Transmittance (%) 0 100 5.0 100 7.7 100 #2 (8 hrs later) #2 (24 hrs later) #2 (48 hrs later) #2 (72 hrs later) Flat Glass Acrylic Resin Plate PVC Plate PC Plate 8.2 17.8 21.0 27.9 40.6 64.5 75.3 86.0 100 89.8 88.5 87.0 45.5 60.6 44.7 49.0 As will be apparent from Table above, it was confirmed that an extremely high antifogging capability could be achieved if the contact angle with water was not greater than 10.degree.. Example 13 Photocatalyst-Containing Silicone Coating This Example is related to the discovery that a coating of a certain high molecular weight compound and containing a photocatalyst is rendered highly hydrophilic when subjected to irradiation with UV light. As substrates, aluminum plates (10 cm square in size) were used. Each of the substrates was first coated with a silicone layer to smooth the surface. To this end, a first component "A" (silica sol) and a second component "B" (trimethoxymethylsilane) of the coating composition "Glaska" marketed by Japan Synthetic Rubber Co. (Tokyo) were mixed with each other in such a manner that the ratio by weight of silica to trimethoxymethylsilane was equal to 3. The resultant coating mixture was applied on each of the aluminum substrates and was subjected to curing at a temperature of 150.degree. C. to obtain a plurality of aluminum substrates (#1 specimens) each coated with a base coating of silicone of 3 .mu.m in thickness. Then, the #1 specimens were coated with a high-molecular-weight coating composition containing a photocatalyst. In order to prevent a film forming element of the coating composition from being degraded by photooxidation action of the photocatalyst, silicone was selected as the film forming element. More specifically, a sol of the anatase form of titania (Nissan Chemical Ind., TA-15) and the first component "A" (silica sol) of the above-mentioned "Glaska" were admixed. After dilution by ethanol, the above-mentioned second component "B" of "Glaska" was further added thereto to prepare a titania containing coating composition. The coating composition was comprised of 3 parts by weight of silica, 1 part by weight of trimethoxymethylsilane, and 4 parts by weight of titania. The coating composition was applied onto the surface of the #1 specimens and was cured at a temperature of 150.degree. C. to obtain #2 specimens coated with a top coating wherein particles of the anatase form of titania were dispersed throughout a coating film of silicone. Then the #2 specimens were subjected to irradiation with UV light for 5 days, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp to obtain #3 specimens. When the contact angle with water of the surface of these specimens was measured by the contact angle meter (made by ERMA), surprisingly the reading of the contact angle meter was less than 3.degree.. The contact angle of the #2 specimens measured prior to UV irradiation was 70.degree.. The contact angle of the #1 specimens as measured was 90.degree.. Then, the #1 specimens were subjected further to irradiation with UV light for 5 days under the same condition as the #2 specimens and the contact angle thereof was measured, the contact angle as measured being 85.degree.. From the foregoing, it has been discovered that, notwithstanding the fact that silicone inherently is substantially hydrophobic, silicone is rendered highly hydrophilic when it contains a photocatalyst and provided that the photocatalyst is photoexcited by irradiation with UV light. Example 14 Raman Spectroscopic Analysis By using a mercury lamp, the #2 specimen of Example 13 was subjected to irradiation with UV light for 2 hours, at a UV intensity of 22.8 mW/cm.sup.2, to obtain #4 specimen. The #2 specimen prior to UV irradiation and the #4 specimen subsequent to UV irradiation were subjected to Raman spectroscopic analysis. For the purposes of comparison, a UV light was irradiated upon the #1 specimen under the same conditions and the specimen was subjected to Raman spectroscopic analysis prior to and subsequent to UV irradiation. Raman spectra are shown in the graph of FIG. 6. In the graph of FIG. 6, the Raman spectra of the #1 specimen prior to and subsequent to UV irradiation are shown by the single curve #1 because they are identical. Referring to the graph of FIG. 6, in the Raman spectrum of the #2 specimen, a dominant peak is noted at the wavenumber 2910 cm.sup.-1 corresponding to the symmetrical stretching of the C--H bond of the sp.sup.3 hybrid orbital and a salient peak is observed at the wavenumber 2970 cm.sup.-1 indicating the inverted symmetrical stretching of the C--H bond of the sp.sup.3 hybrid orbital. It can therefore be concluded that the C--H bonds are present in the #2 specimen. In the Raman spectrum of the #4 specimen, no peak is found at the wavenumbers 2910 cm.sup.-1 and 2970 cm.sup.-1. Instead, a broad absorption band peaking at the wavenumber 3200 cm.sup.-1 and corresponding to the symmetrical stretching of the O--H bond is observed. It is therefore concluded that, in the #4 specimen, there is no C--H bond but, instead, the O--H bonds are present. In contrast, in the Raman spectrum of the #1 specimen, a dominant peak at the wavenumber 2910 cm.sup.-1 corresponding to the symmetrical stretching of the C--H bond of the sp.sup.3 hybrid orbital as well as a salient peak at the wavenumber 2970 cm.sup.-1 corresponding to the inverted symmetrical stretching of the C--H bond of the sp.sup.3 hybrid orbital are noted throughout the points of time prior to and subsequent to UV irradiation. Accordingly, it is confirmed that the C--H bonds are present in the #1 specimen. From the foregoing, it is considered that, when silicone which contains a photocatalyst is subjected to irradiation with UV light, the organic groups bonded to the silicon atoms of the silicone molecules as represented by the general formula (1) below are substituted with the hydroxyl groups under the action of the photocatalyst so that a derivative of silicone is formed at the surface as shown by the formula (2). ##STR1## where R represents alkyl or aryl group. ##STR2## Example 15 Antifogging Plastic Plate--Antifogging Coating of Photocatalyst-Containing Silicone The surface of a plastic substrate was first coated with a silicone layer to prevent the substrate from being degraded by the photocatalyst. To this end, a coating solution was prepared in a manner similar to Example 13 by admixing the first and second components "A" and "B" of the above-mentioned "Glaska" of Japan Synthetic Rubber Co. such that the ratio by weight of silica to trimethoxymethylsilane was equal to 3. The coating solution was applied on the surface of 10 cm-square acrylic resin plates, and each plate was then cured at a temperature of 100.degree. C. to obtain a plurality of acrylic resin plates (1 specimens) each coated with a base coating of silicone of 5 .mu.m in thickness. Next, a sol of the anatase form of titania (Nissan Chemical Ind., TA-15) and the first component "A" of the above-mentioned "Glaska" were admixed and, after diluted by ethanol, the second component "B" of "Glaska" was added thereto to prepare four kinds of coating solutions having different compositions. The compositions of these coating solutions were such that the ratio by weight of titania to the sum of titania plus silica plus trimethoxymethylsilane was equal to 5%, 10%, 50%, and 80%, respectively. These coating solutions were applied, respectively, onto the surface of the acrylic resin plates coated with the silicone layer and were cured at a temperature of 100.degree. C. to obtain #2-#5 specimens each coated with a top coating wherein particles of the anatase form of titania were dispersed throughout a coating film of silicone. Then the #1-#5 specimens were subjected to irradiation with UV light by a BLB fluorescent lamp for maximum 200 hours, at a UV intensity of 0.5 mW/cm.sup.2, and the contact angle with water of the surface of these specimens was measured by the contact angle meter (made by ERMA) at different time points to see the variation in response to time of the contact angle. The results are shown in the graph of FIG. 7. As will be understood from the graph of FIG. 7, in the #1 specimen, which was not provided with A titania-containing coating, no appreciable change in the contact angle with water resulted from UV irradiation. In contrast, in each of the #2-#5 specimens (each of which had a titania-containing top coating), it will be noted that upon UV irradiation the surface was rendered hydrophilic to the degree that the contact angle with water became less than 10.degree.. In particular, it will be understood that, in the #3-#5 specimens wherein the titania content was greater than 10% by weight, the contact angle with water became less than 3.degree.. Furthermore, it will be noted that in the #4 and #5 specimens having a titania contents of 50% by weight and 80% by weight, respectively, the contact angle with water became less than 3.degree. within a relatively short time of beginning UV irradiation. When a blow of breath was blown upon the #4 specimen, no formation of fog was observed. After keeping the #4 specimen in the dark for 2 weeks, the contact angle with water was measured by the contact angle meter (CA-X150) and was found to be less than 3.degree.. Example 16 Pencil Scratch Test A pencil scratch test was conducted to ascertain the abrasion resistance of the titania-containing top coating. In a manner similar to Example 15, a plurality of 10 cm-square acrylic resin plates were first coated with a base coating of silicone of 5 .mu.m in thickness and were then coated with a top coating having varying titania content. In these plates, the titania content of the top coating was 50% by weight, 60% by weight, and 90% by weight, respectively. According to the method H8602 of the Japanese Industrial Standard (JIS), the surface of the specimens was scratched by various pencil leads to find the hardest pencil lead by which the top coating was peeled off. A similar test was also conducted for a specimen which was coated only with the base coating. The results are shown in the graph of FIG. 8. The top coating having a titania content of 90% by weight was peeled off by a pencil lead of hardness 5B, but the top coating having a titania content of 60% by weight was able to withstand a pencil lead of hardness H and showed an adequate abrasion resistance. The abrasion resistance of the top coating apparently increases with decreasing titania content. Example 17 Effect of coating Thickness In a manner similar to Example 13, a plurality of 10 cm-square aluminum plates were first coated with a base coating of silicone of 5 .mu.m in thickness and were then coated with an anatase-containing top coating of varying thickness to obtain a plurality of specimens. The thickness of the top coating of the #1 specimen was 0.003 .mu.m, the thickness of the top coating of the #2 specimen being 0.1 .mu.m, the thickness of the top coating of the 43 specimen being 0.2 .mu.m, the thickness of the top coating of the #4 specimen being 0.6 .mu.m, and the thickness of the top coating of the #5 specimen being 2.5 .mu.m. While subjecting the respective specimens to irradiation with UV light, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp, the variation in response to time of the contact angle with water of the surface of the specimens was measured by the contact angle meter (made by ERMA). The results are shown in the graph of FIG. 9. As will be apparent from the graph of FIG. 9, regardless of the thickness of the coating, the surface of the respective specimens was rendered highly hydrophilic within 50 hours of UV irradiation to the degree that the contact angle with water became less than 3.degree.. It will be noted in particular that, even with the titania-containing top coating of the thickness of less than 0.2 .mu.m, a sufficient photocatalytic activity was achieved to the degree that the top coating surface was rendered highly hydrophilic. In this regard, it is known that a transparent layer is colored due to interference of light when the thickness of the layer exceeds 0.2 .mu.m. This Example illustrates that, by limiting the thickness of the top coating to 0.2 .mu.m or less, the surface of the top coating can be made highly hydrophilic while preventing coloring thereof due to interference of light. Next, the #1-#5 specimens were tested for the capability thereof to photodecompose methyl mercaptan. Each specimen was placed, separately, in a desiccator of 11 liters in volume made of UV permeable quartz glass, and nitrogen gas containing methyl mercaptan was introduced therein in such a manner that the methyl mercaptan concentration equaled 3 ppm. A 4 W BLB fluorescent lamp was placed within the desiccator at a distance of 8 cm from the specimen to irradiate the specimen, at a UV intensity of 0.3 mW/cm.sup.2. By sampling gas in the desiccator 30 minutes later, the methyl mercaptan concentration was measured by gas chromatography and the removal rate of methyl mercaptan was calculated. The results are shown in the graph of FIG. 10. The graph of FIG. 10 indicates that the photodecomposition capability of the photocatalytic coating vis-a-vis methyl mercaptan increases with increasing coating thickness. It is found that the photocatalytic photodecomposition capability was clearly affected by the thickness of the photocatalytic layer. In view of the results shown in FIG. 9, it seems that the photocatalytic superhydrophilification process is not necessarily identical with the photocatalytic redox process known hitherto in the field of photocatalyst. Example 18 Highly Hydrophilic Photocatalytic Coating of Titania-Containing Silicone In a manner similar to Example 13, a 10 cm-square aluminum plate was first coated with a base coating of silicone of 5 .mu.m in thickness. Then, a sol of the anatase form of titania (Nissan Chemical Ind., TA-15) and the second component "B" (trimethoxymethylsilane)of the above-mentioned "Glaska" were admixed with each other and the mixture was diluted by ethanol to prepare a coating composition containing titania. The ratio by weight of trimethoxymethylsilane to titania was equal to 1. The coating composition was applied onto the surface of the aluminum plate and was cured at a temperature of 150.degree. C. to form a top coating wherein particles of the anatase form of titania were dispersed throughout a coating film of silicone. The thickness of the coating was 0.1 .mu.m. Then the specimen was subjected to irradiation with UV light for a day, at a UV intensity of 0.5 mW/cm.sup.2, by using a BLB fluorescent lamp. When the contact angle with water of the surface of this specimen was measured by the contact angle meter (CA-X150), the reading of contact angle was 0.degree.. The specimen was kept in the dark for 3 weeks and the contact angle with water was measured each week. The measured contact angle is shown in Table 3. TABLE 3 immed. after irradiation 1 week later 0.degree. 2.degree. 2 weeks later 1.degree. 3 weeks later 3.degree. As will be understood from Table 3, once the surface has been superhydrophilified, superhydrophilicity will be sustained for a substantially long time period even in the absence of photoexcitation. Example 19 Antibacterial Enhancer--Ag-Added Photocatalyst In a manner similar to Example 1, a thin film of amorphous silica and a thin film of amorphous titania were formed in sequence on the surface of a 10 cm-square soda-lime glass plate and the glass plate was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form of titania, whereby #1 specimen was obtained. Then an aqueous solution containing 1% by weight of silver lactate was applied onto the surface of the #1 specimen, and the specimen was subjected to irradiation with UV light for one minute by operating a 20 W BLB fluorescent lamp positioned at a distance of 20 cm from the specimen whereby #2 specimen was obtained. Upon UV irradiation, silver lactate underwent photoreduction to form silver deposit and the surface of the specimen was rendered hydrophilic under the photocatalytic action of titania. The #1 specimen was also subjected to UV irradiation under the same conditions. When the contact angle with water of the #1 and #2 specimens was measured by the contact angle meter (made by ERMA), the contact angle in both specimens was less than 3.degree.. When a blow of breath was blown upon these specimens, no formation of fog was observed. For the purposes of comparison a substrate of soda-lime glass, without coating, was tested, and it was found that the contact angle with water was 50.degree. and a fog was readily formed upon blowing of breath. Then, the #1 and #2 specimens as well as the uncoated soda-lime glass plate were tested for antibacterial capability. A liquid culture prepared by shake cultivating colibacillus (Escherichia coli W3110 stock) for a night was subjected to centrifugal washing and was diluted with sterilized distilled water by 10,000 times to prepare a bacteria containing liquid. 0.15 ml of the bacteria containing liquid (equivalent to 10000-50000 CFU) was dripped on three glass slides which were then brought into intimate contact with the #1 and #2 specimens and the uncoated soda-lime glass plate, respectively, all of which had previously been sterilized by 70% ethanol. The specimens and the uncoated plate were then subjected to irradiation from a white fluorescent lamp placed in front of the glass slides for 30 minutes, at an intensity of 3500 lux. Thereafter, the bacteria containing liquid of respective specimens was wiped by a sterilized gauze and was recovered in 10 ml of physiological saline and the liquid thus recovered was applied for inoculation on a nutrient agar plate for culture at 37.degree. C. for a day. Thereafter, the colonies of colibacillus formed on the culture was counted to calculate the survival rate of colibacillus. The result was that in the #1 specimen and the soda-lime glass plate the survival rate of colibacillus was greater than 70%, but the survival rate was less than 10% in the #2 specimen. This experiment demonstrates that, when the photocatalyst is doped with Ag, the surface of the substrate is not only rendered highly hydrophilic but also becomes antibacterial. Example 20 Antibacterial Enhancer--Cu-Added Photocatalyst In a manner similar to Example 1, a thin film of amorphous silica was formed on the surface of each of a plurality of 10 cm-square soda-lime glass plates to obtain a plurality of #1 specimens. Then, similar to Example 1, a thin film of amorphous titania was formed on the surface of one #1 specimen which was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form titania. Then an ethanol solution containing 1 weight percent of copper acetate was applied by spray coating onto the surface of one specimen and, after drying, the specimen was subjected to irradiation with UV light for one minute by a 20 W BLB fluorescent lamp positioned at a distance of 20 cm from the specimen, to thereby subject copper acetate to photoreduction deposition and, thus, to obtain a #2 specimen wherein crystals of titania were doped with copper. As inspected by the eye, the #2 specimen presented an adequate light transmittance. A soda-lime glass plate as well as the #2 specimen and the #1 specimen (without titania coating) immediately after fabrication were tested for antifogging capability and the contact angle with water measured. The antifogging test was done by blowing a blow of breath upon the specimen to produce a fog on the specimen surface and by inspecting for the presence or absence of particles of moisture condensate, using a microscope. The contact angle was measured by the contact angle meter (made by ERMA). The results are shown in Table 4. TABLE 4 Immediately After Preparation of Specimen #2 Specimen #1 Specimen Soda-Lime Glass Contact Angle with Water (.degree.) 10 Antifogging Property no fog 9 50 no fog fogged Further, after being subjected to irradiation with UV light for a month, at a UV intensity of 0.5 mW/cm.sup.2, by a BLB fluorescent lamp, the #2 and #1 specimens and the soda-lime glass plate were tested in a similar manner for antifogging capability and contact angle. The results are shown in Table 5. TABLE 5 After 1 Month of UV Irradiation Contact Angle with Antifogging Water (.degree.) Property #2 Specimen 3 no fog #1 Specimen 49 fogged Soda-Lime Glass 53 fogged Then, the #2 and #1 specimens immediately after preparation and the soda-lime glass plate were tested for antibacterial capability in a manner similar to that described in Example 19. The result was that in the soda-lime glass plate and the #1 specimen the survival rate of colibacillus was greater than 70%, but the survival rate was less than 10% in the #2 specimen. Next, the #2 and #1 specimens immediately after preparation, and the uncoated soda-lime glass plate, were tested for deodorizing performance. Each specimen was placed in a desiccator of 11 liters in volume made of UV permeable quartz glass and nitrogen gas containing methyl mercaptan was introduced therein in such a manner that the methyl mercaptan concentration equaled 3 ppm. In each case, a 4 W BLB fluorescent lamp was placed within the desiccator at a distance of 8 cm from the specimen to irradiate the specimen, at a UV intensity of 0.3 mW/cm.sup.2. By sampling gas in the desiccator 30 minutes later, the methyl mercaptan concentration was measured by gas chromatography and the removal rate of methyl mercaptan was calculated. With the #1 specimen and the soda-lime glass plate, the removal rate of methyl mercaptan was less than 10%. In contrast, the removal rate of the #2 specimen was more than 90%, so that a good deodorizing performance was achieved. Example 21 Antibacterial Enhancer--Cu-Added Photocatalyst The first and second components "A" (silica sol) and "B" (trimethoxymethylsilane) of "Glaska" of Japan Synthetic Rubber Co. were admixed such that the ratio by weight of silica to trimethoxymethylsilane was equal to 3, and the mixture was applied on the surface of a 10 cm-square acrylic resin plate, followed by curing at a temperature of 100.degree. C. to obtain an acrylic resin plate coated with a base coating of silicone of 3 .mu.m in thickness. Then, a sol of the anatase form of titania (TA-15) and an aqueous solution containing 3 weight percent of copper acetate were mixed and, after adding further the first component "A" (silica sol) of "Glaska" thereto, the mixture was diluted by propanol. Then the second component "B" of "Glaska" was further added to prepare a titania-containing coating composition. The coating composition was comprised of 3 parts by weight of silica, 1 part by weight of trimethoxymethylsilane, 4 parts by weight of titania, and 0.08 parts by weight of copper acetate in terms of metallic copper. The coating composition was applied onto the surface of the acrylic resin plate and was cured at a temperature of 100.degree. C. to form a top coating. Then the specimen was subjected to irradiation with UV light for 5 days, at a UV intensity of 0.5 mW/cm.sup.2 by using a BLB fluorescent lamp to obtain #1 specimen. The #1 specimen and the acrylic resin plate were investigated for antifogging capability, contact angle with water, antibacterial performance and deodorizing function, in a manner similar to Example 20. In the acrylic resin plate, the contact angle with water was 70.degree. and a fog was formed as a blow of breath was blown upon. In the #1 specimen, however, the contact angle with water was 3-9.degree. and formation of fog did not occur. With regard to antibacterial property, in the acrylic resin plate the survival rate of colibacillus was greater than 70%, whereas the survival rate was less than 10% in the #1 specimen. Regarding the deodorizing property, while the removal rate of methyl mercaptan by the acrylic resin plate was less than 10%, the removal rate by the #1 specimen was more than 90%. Example 22 Photo-Redox Activity Enhancer--Pt-Added Photocatalyst In a manner similar to Example 1, a thin film of amorphous silica and then a thin film of amorphous titania were formed on the surface of a 10 cm-square soda-lime glass plate and the glass plate was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form titania. Then, 1 ml of aqueous solution of chloroplatinic acid 6-hydrate H.sub.2 PtCl.sub.6.6H.sub.2 O containing 0.1 weight percent of platinum was applied onto the specimen which was then subjected to irradiation with UV light for one minute, at a UV intensity of 0.5 mW/cm.sup.2 by a BLB fluorescent lamp to thereby form deposit of platinum by photoreduction of chloroplatinic acid hexahydrate to obtain a specimen wherein crystals of titania were doped with platinum. The specimen thus obtained was left as such for a day and was thereafter subjected to irradiation with UV light for a day, at a UV intensity of 0.5 mW/cm.sup.2 by using a BLB fluorescent lamp. The contact angle measured after UV irradiation was 0.degree.. Furthermore, the removal rate of methyl mercaptan as measured and calculated in a manner similar to Example 20 was 98%. Example 23 Self-Cleaning and Antifogging Capability The #2 specimen of Example 13 was subjected to irradiation with UV light for 10 hours, at a UV intensity of 0.5 mW/cm.sup.2 by using a BLB fluorescent lamp to obtain #3 specimen. When the contact angle with water of the surface of this specimen was measured by the contact angle meter (made by ERMA), the reading of the contact angle meter was less than 3.degree.. An outdoor accelerated fouling test apparatus as shown in FIGS. 11A and 11B was installed atop of a building located in chigasaki City. Referring to FIGS. 11A and 11B, this apparatus includes an inclined specimen mounting surface 22 supported by a frame 20 and adapted to affix specimens 24 thereto. A forwardly slanted roof 26 is fixed at the top of the frame. The roof is made of corrugated plastic sheet and is designed to permit collected rain water to flow down in a striped pattern along the surface of the specimens 24 affixed on the specimen mounting surface 22. The #3 specimens, the #1 specimens of Example 13, and the #2 specimens of Example 13 were mounted to the specimen mounting surface 22 of the apparatus and were exposed to the weather conditions for 9 days starting from Jun. 12, 1995. The weather and the amount of rain fall during this period were as shown in Table 6. TABLE 6 Date June 12 June 13 June June June June June June June 14 15 16 17 18 19 20 Weather cloudy heavy rain Rainfall (mm) 0.0 53.0 cloudy/rain cloudy/fair cloudy fair/cloudy fair to cloudy rain to cloudy cly/heavy rain 20.5 0.0 0.0 0.0 0.0 1.0 56.0 Shining Hours 0 0 0 3.9 0.2 9.6 7.0 0.2 2.4 When inspected on June 14, dirt or smudge of a striped pattern was observed on the surface of the #1 specimen. Presumably, this is because during heavy rainfall on the preceding day the airborne hydrophobic contaminants such as combustion products like carbon black and city grime were carried by rain and were allowed to deposit on the specimen surface as rain water flowed down along the surface. In contrast, no dirt or smudge was observed in the #3 specimen. Believably, this is because, since the specimen surface was rendered highly hydrophilic, the hydrophobic contaminants were unable to adhere onto the surface as rain water containing contaminants flowed down and further because the contaminants were washed away by rainfall. In the #2 specimen, dirt or smudge of a mottled pattern was observed. This is probably because, after the #2 specimen which had not been subjected to UV irradiation was mounted to the testing apparatus, the photocatalytic coating thereof was not yet exposed to UV light in the sunlight to a satisfactory degree so that the surface was unevenly hydrophilified. When inspected on June 20, a smudge of a vertically striped pattern was remarkably noticed on the surface of the #1 specimen which was not provided with the photocatalytic coating. Conversely, no smudge was observed on the #3 and #2 specimens provided with the photocatalytic coating. The contact angle with water as measured was 70.degree. for the #1 specimen and was less than 3.degree. for the #2 and #3 specimens. The fact that the contact angle of the #2 specimen became less than 3.degree. demonstrates that, upon irradiation by TV light contained in the sunlight, the organic groups bonded to the silicon atoms of the silicone molecules of the top coating were substituted with hydroxyl groups under the photocatalytic action so that the top coating was rendered highly hydrophilic. It was also noted that in the #3 specimen a high degree of hydrophilicity was sustained by irradiation of the sunlight. Example 24 Color Difference Test Prior to and 1 month after mounting to the outdoor accelerated fouling test apparatus, the #1 and #2 specimens of Example 23 were tested by a color difference meter (Tokyo Denshoku) to measure a color difference. In compliance with the Japanese Industrial Standard (JIS) H0201, the color difference was indicated by the .DELTA.E* index. The variation in the color difference after mounting to the accelerated fouling test apparatus is shown in Table 7. #1 Specimen #2 Specimen TABLE 7 Striped Area 4.1 0.8 Background 1.1 0.5 As will be noted from Table 7, in the #1 specimen void of the photocatalytic coating, a large amount of smudge was caused to adhere to the vertical striped area corresponding to the flow path of rainwater, as compared with the #2 specimen provided with the photocatalytic coating. It will also be recognized that, between the #2 and #1 specimens, there was a substantial difference in the degree of fouling of the background area. Example 25 Cleansing Capability for Oil Stains A quantity of oleic acid was applied on the surface of the #1 and #3 specimens of Example 23, respectively, and the specimens were then immersed in water in a cistern with the specimen surface held in a horizontal position. In the #1 specimen, oleic acid remained adhered to the specimen surface. In contrast, in the #3 specimen, oleic acid became rounded to form oil droplets which were then released from the surface of the specimen to rise to the top of the water. In this manner, it was confirmed that, in the case that the surface of a substrate was coated with a photocatalytic top coating, the surface was maintained hydrophilic so that, when soaked in water, oily stains were readily released away from the surface whereby the surface was cleansed. This Example illustrates that a tableware, for instance, fouled by oil or fat can be readily cleansed only by soaking it in water without recourse to a detergent, provided that the surface thereof is provided with a photocatalytic coating and if the photocatalyst is photoexcited by UV irradiation. Example 26 Drying of Water Wet Surface The surface of the #1 and #3 specimens of Example 23 were wetted with water and the specimens were left outdoors on a fair day to subject them to natural drying. The ambient temperature was about 25.degree. C. As the #1 specimen was inspected 30 minutes later, water droplets still remained on the surface. In contrast, it was found that the surface of the #3 specimen was completely dried. It is considered that in the #3 specimen provided with the photocatalytic coating, the adherent water droplets were caused to spread into a uniform film of water and for this reason drying was accelerated. This Example illustrates the possibility that an eyeglass lens or automotive windshield wetted with water may be promptly dried. Example 27 Tile with Highly Hydrophilic Surface--Coating of Sintered Titania and Silica A sol of the anatase form of titania (Ishihara Industries of Osaka, STS-11) and a sol of colloidal silica (Nissan Chemical Ind., "Snowtex O") were admixed at a ratio by mol of 88:12 in terms of solid matter and the mixture was applied by spray coating on the surface of a glazed tile (Toto Ltd., AB02E01) of 15 cm square in size, followed by sintering for 1 hour at a temperature of 800.degree. C. to obtain a specimen covered by a coating comprised of titania and silica. The thickness of the coating was 0.3 .mu.m. The contact angle with water immediately after sintering was 5.degree.. The specimen was kept in the dark for a week but the contact angle measured thereafter was still 5.degree.. As the specimen surface was subjected to irradiation with UV light for 1 day, at a UV intensity of 0.03 mW/cm.sup.2 by using a BLB fluorescent lamp, the contact angle with water became 0.degree.. Example 28 Coating of Sintered Titania and Silica--Hydrophilification under Room Light A sol of the anatase form of titania (STS-11) and a sol of colloidal silica (Nissan Chemical Ind., "Snowtex 20") were admixed at a ratio by mol of 80:20 in terms of solid matter and the mixture was applied by spray coating on the surface of a 15 cm-square glazed tile (AB02E01), followed by sintering for 1 hour at a temperature of 800.degree. C. to obtain a specimen covered by a coating comprised of titania and silica. The thickness of the coating was 0.3 .mu.m. The contact angle with water immediately after sintering was 5.degree.. The contact angle with water as measured after keeping the specimen in the dark for 2 weeks was 14.degree.. As the specimen surface was subjected to irradiation with UV light for 1 day, at a UV intensity of 0.004 mW/cm.sup.2 by a white fluorescent lamp, the contact angle with water became 4.degree.. Accordingly, it was found that the photocatalytic coating was rendered hydrophilic to a satisfactory degree even under indoor illumination. Example 29 Coating of Sintered Titania and Silica--Silica Content A sol of the anatase form of titania (STS-11) and a sol of colloidal silica (Nissan Chemical Ind., "Snowtex 20") were admixed at a varying ratio to obtain a plurality of suspensions having a ratio by mol of silica to the solid matter of the suspension of 0%, 5%, 10%, 15%, 20%, 25% and 30%, respectively. 0.08g of each suspension was uniformly applied by spray coating on the surface of a 15 cm-square glazed tile (AB02E01) and each tile was fired for 1 hour at a temperature of 800.degree. C. to obtain a plurality of specimens each covered by a coating comprised of titania and silica. The contact angle with water immediately after sintering of the respective specimens was as shown in the graph of FIG. 12. As will be apparent from the graph of FIG. 12, the initial contact angle was lowered by addition of silica. The contact angle with water as measured after keeping the specimen in the dark for 8 days was plotted in the graph of FIG. 13. As will be noted by comparing the graph of FIG. 12 with the graph of FIG. 13, the loss of hydrophilicity resulting from keeping the specimens in the dark is small in the specimens containing more than 10%, in the ratio by mol, of silica. Thereafter, the specimens were subjected to irradiation with UV light for 2 days, at a UV intensity of 0.03 mW/cm.sup.2 by using a BLB fluorescent lamp. The contact angle with water after irradiation is shown in the graph of FIG. 14. It will be noted from the graph that upon UV irradiation hydrophilicity is readily recovered in the case where silica is added to titania. Then the specimens were kept in the dark for further a days and the contact angle with water was measured. The results are shown in FIG. 15. It will be noted from the graph that the loss of hydrophilicity resulting from keeping the specimens in the dark after UV irradiation is small in the case where silica is added to titania. A pencil scratch test was carried out to examine the abrasion resistance of the sintered film comprised of titania and silica. The results are shown in the graph of FIG. 16. It will be understood that the abrasion resistivity is increased with increasing silica content. Example 30 Sludge Test A mixture of a sol of the anatase form of titania (STS-11) and a sol of colloidal silica (Snowtex 20) and having a silica content of 10% by weight in terms of solid matter was applied to a 15 cm-square glazed tile (AB02E01) in an amount of 4.5 mg in terms of solid matter and the tile was then calcined for 10 minutes at a temperature of 880.degree. C. The specimen was then subjected to irradiation with UV light for 3 hours, at a UV intensity of 0.5 mW/cm.sup.2 by using a BLB fluorescent lamp to obtain #1 specimen. The contact angle with water of the #1 specimen and the glazed tile (AB02E01) as such was 0.degree. and 30.degree., respectively. A mixture of powders of 64.3% by weight of yellow ochre, 21.4% by weight of calcined Kanto loam clay, 4.8% by weight of hydrophobic carbon black, 4.8% by weight of silica powder, and 4.7% by weight of hydrophilic carbon black was suspended in water at a concentration of 1.05 g/l to prepare a slurry. 150 ml of the thus prepared slurry was caused to flow down along the surface of the #1 specimen and the glazed tile (AB02E01) held inclined at 45.degree., followed by drying for 15 minutes, and 150 ml of distilled water was thereafter caused to flow down, followed by drying for 15 minutes, the cycle of the above-mentioned sequences being repeated for 25 times. A change in color difference and in glossiness after the sludge test was measured. The measurement of the glossiness was carried out according to the method laid down by the Japanese Industrial Standard (JIS) Z8741 and the variation in the glossiness was obtained by dividing the glossiness after testing by the glossiness before testing. The results are given in Table 8. TABLE 8 #1 Specimen Tile (AB02E01) Contact Angle (.degree.) 0 Color Diff. Change 0.7 Glossiness Change 93.4% 30 5.6 74.1% Example 31 Relationship between Contact Angle with Water and Self-Cleaning and Antifouling Capability Various specimens were subjected to a sludge test in a manner similar to Example 30. The tested specimens included the #1 specimen of Example 30, #2 specimen having a copper-doped titania coating, the glazed tile (AB02E01), an acrylic resin plate, an artificial marble plate (Toto Ltd., ML03) made of polyester resin matrix, and a polytetrafluoroethylene (PTFE) plate. The #2 specimen was prepared by spray coating 0.3g of an aqueous solution of copper acetate monohydrate having a copper concentration of 50 .mu.mol/g on the #1 specimen of Example 30 and, after drying, subjecting the specimen to irradiation with UV light for 10 minutes, at a UV intensity of 0.4 mW/cm.sup.2 by a BLB fluorescent lamp to thereby subject copper acetate monohydrate to photoreduction deposition. The results of the sludge test are shown in Table 9. TABLE 9 Contact Angle Color Difference Specimen with Water (.degree.) Change #1 Specimen 0.0 0.7 #2 Specimen 4.0 2.0 Glazed Tile 19.4 4.6 Acrylic Plate 50.9 4.5 Artif. Marble 54.8 3.2 PTFE Plate 105.1 0.9 Glossiness Change (%) 93.8 81.5 68.3 69.3 85.2 98.2 Furthermore, various specimens were subjected for a period of a month to an accelerated fouling test similar to Example 23. The specimens used included the #1 specimen of Example 30, the glazed tile (AB02E01), an acrylic resin plate, an aluminum plate covered by a base coating of silicone in a manner similar to Example 13, and a PTFE plate. The results of the accelerated tests are shown in Table 10 wherein, similar to Example 24, the change in the color difference represents that of the vertical striped area of the specimens. TABLE 10 Specimen #1 Specimen Glazed Tile Acrylic Plate Silicone Coated PTFE Plate Contact Angle with Water (.degree.) 0.0 19.4 50.9 90.0 105.1 Color Difference Change 0.9 1.5 2.3 4.2 7.8 To facilitate understanding, the contact angle with water as well as the variation in the color difference are plotted in the graph of FIG. 17. In the graph of FIG. 17, the curve A indicates the relationship between the contact angle with water and the color difference change caused by the contaminants such as airborne combustion products like carbon black and city grime as a result of the accelerated fouling test, with the curve B representing the relationship between the contact angle with water and the color difference change caused by sludge as a result of the sludge test. Referring to the graph of FIG. 17, as the contact angle with water of the substrate increases, the dirt or stain due to combustion products and city grime becomes more conspicuous, as will be readily understood from the curve A. This is because the contaminants such as combustion products and city grime are generally hydrophobic and, hence, are apt to adhere to a hydrophobic surface. In contrast, the curve B illustrates that the dirt or stain due to sludge peaks when the contact angle with water is in the range of 20-50.degree.. This is because the inorganic substances such as loam and soil inherently have a hyrdophilicity on the order of 20-50.degree. in terms of the contact angle with water so that they are apt to adhere to a surface having a similar hyrdophilicity. It will therefore be understood that, by rendering the surface hyrdophilic to the degree that the contact angle with water is less than 20.degree. or, alternatively, by rendering the surface hyrdophobic to the degree that the contact angle with water is greater than 60.degree., the adherence of the inorganic substances to a surface can be prevented. The reason why fouling by sludge is reduced as the contact angle with water is less than 20.degree. is that, when the surface is rendered highly hydrophilic to the degree that the contact angle with water becomes less than 20.degree., the affinity of the surface for water exceeds the affinity for inorganic substances so that adherence of inorganic substances is blocked by water which preferentially adheres to the surface and any inorganic substances that have adhered to or are tending to adhere to the surface are readily washed away by water. It will be noted from the foregoing that, in order to prevent both the hydrophobic and hydrophilic substances from adhering to the surface of a building and the like, or in order to ensure that dirt or smudge deposited on the surface is washed away by rain water so as to permit the surface to be self-cleaned, it is desirable to modify the surface to present a contact angle with water of less than 20.degree., preferably less than 10.degree., more preferably less than 5.degree.. Example 32 Coating of Sintered Titania and Tin Oxide--Glazed Tile A sol of the anatase form of titania (STS-11) and a sol of tin oxide (Taki Chemical K.K. of Kakogawa City, Hyogo-Prefecture; mean crystallite size of 3.5 nm) were admixed at various blending ratio (percent by weight of tin oxide to the sum of titania plus tin oxide) shown in Table 11 and the mixtures were applied by spray coating on the surface of 15 cm-square glazed tiles (ABO2E01), followed by sintering for 10 minutes at a temperature either of 750.degree. C. or 800.degree. C. to obtain #1-#6 specimens. After sintering, the #2, #4, #5 and #6 specimens were further doped with silver by applying thereon an aqueous solution containing 1 weight percent of silver nitrate and by subjecting silver nitrate to photoreduction deposition. In addition, #7-#9 specimens were further prepared by applying onto the glazed tiles only a sol of tin oxide or a sol of titania and by sintering. After sintering, the #7 and #9 specimens were further doped with silver. Each specimen was kept in the dark for a week and was thereafter subjected to irradiation with UV light for 3 days, at a UV intensity of 0.3 mW/cm.sup.2 by using a BLB fluorescent lamp whereupon the contact angle with water was measured. The results are shown in Table 11. TABLE 11 Specimen (.degree.) SnO.sub.2 Ratio Sintering (wt %) Temp. (.degree. C.) Ag Contact Angle #1 1 800 None 0 #2 #3 #4 #5 #6 #7 #8 #9 5 15 15 50 95 100 0 0 800 800 750 750 800 750 800 800 Added None Added Added Added Added None Added 0 0 0 0 5 8 11 14 As will be apparent from Table 11, in the #8 and #9 specimens which were coated only with titania, the contact angle with water exceeded 10.degree.. This is because the alkaline network-modifier ions such as sodium ions diffused from the glaze into the titania coating during sintering whereby the photocatalytic activity of anatase was hindered. In contrast, it will be noted that, in the #1-#6 specimens wherein SnO.sub.2 were blended, the surface was hydrophilified to a high degree. As shown by the #7 specimen, tin oxide which is a semiconductor photocatalyst is effective in rendering the surface hydrophilic in a manner similar to titania. Although the reason therefor is not clear, this Example illustrates that the effect of diffusion of the alkaline network-modifier ions can be overcome by adding tin oxide to titania. Example 33 Sintered Titania Coating and Diffusion Prevention Layer--Glazed Tile Tetraethoxysilane (marketed by Colcoat, "Ethyl 28") was applied by spray coating on the surface of a 15 cm-square glazed tile (AB02E01) which was then held at a temperature of about 150.degree. C. for about 20 minutes to subject tetraethoxysilane to hydrolysis and dehydration polymerization whereby a coating of amorphous silica was formed on the surface of the glazed tile. Then, a sol of the anatase form of titania (STS-11) was applied by spray coating on the surface of the tile which was then fired for an hour at a temperature of 800.degree. C. The thus obtained specimen, as well as the #8 specimen of Example 32 tested for the purposes of comparison, were kept in the dark for a week and were then subjected to irradiation with UV light for 1 day, at a UV intensity of 0.3 mW/cm.sup.2 by using a BLB fluorescent lamp whereupon the contact angle with water was measured. In contrast to the contact angle with water being 12.degree. in the #8 specimen of Example 32, the specimen provided with the intervening layer of amorphous silica was hydrophilified to the degree that the contact angle with water became less than 3.degree.. It is therefore considered that the layer of amorphous silica is effective in preventing diffusion of the alkaline network-modifier ions being present in the glaze layer. Example 34 Amorphous Titania Calcination Coating and Diffusion Prevention Layer--Glazed Tile In a manner similar to Example 1, a thin film of amorphous silica and then a thin film of amorphous titania were formed in sequence on the surface of a 15 cm-square glazed tile (AB02E01). The tile was then calcined at a temperature of 500.degree. C. to transform amorphous titania into the anatase form titania. The specimen thus obtained was kept in the dark for several days and was then subjected to irradiation with UV light for 1 day, at a UV intensity of 0.5 mW/cm.sup.2 by using a BLB fluorescent lamp. The contact angle with water of the resultant specimen as measured was 0.degree.. Similar to Example 33, it is considered that the layer of amorphous silica is effective in rendering the surface of a tile highly hydrophilic. Example 35 Glazed Tile--Cleansing Capability for Oil Stains A quantity of oleic acid was applied on the surface of the #1 specimen of Example 30. When the specimen was then immersed in water in a cistern with the specimen surface held in a horizontal position, oleic acid became rounded to form oil droplets which were then released from the surface of the tile to ascend to the top of the water. This Example also illustrates that a surface of pottery, such as tile and tableware, fouled by oil or fat can be readily cleansed merely by soaking the object in water or by wetting it with water, provided that the surface thereof is provided with a photocatalytic coating and provided that the photocatalyst is photoexcited by UV irradiation. Example 36 Glass--Cleansing Capability for Oil Stains In a manner similar to Example 1, a thin film of amorphous silica and then a thin film of amorphous titania were formed in sequence on the surface of a 10 cm-square soda-lime glass plate. The glass plate was then fired at a temperature of 500.degree. C. to transform amorphous titania into the anatase form titania. A quantity of oleic acid was applied on the surface of the glass plate. As the glass plate was then immersed in water in a cistern with the surface held in a horizontal position, oleic acid became rounded to form oil droplets which were then released from the surface of the glass plate and floated. Example 37 Glass--Self-Cleaning and Antifouling Capability The specimen of Example 36 was subjected for a month to an accelerated fouling test similar to Example 23. When inspected by the eye a month later, no smudge of a vertically striped pattern was observed. Example 38 Glazed Tile--Antibacterial Enhancer (Ag Doping) A coating comprised of titania and silica was formed on the surface of a 15 cm-square glazed tile (AB02E01) in a manner similar to Example 27. Then an aqueous solution containing 1 weight percent of silver lactate was applied onto the surface of the tile which was then subjected to irradiation with UV light of a BLB fluorescent lamp to thereby subject silver lactate to photoreduction to form a silver deposit whereby a specimen coated with silver doped titania was obtained. The contact angle with water as measured was 0.degree.. When the tile was then tested for the antibacterial function in a manner similar to Example 19, the survival rate of colibacillus was less than 10%. Example 39 Glazed Tile--Antibacterial Enhancer (Cu Doping) A coating comprised of titania and silica was formed on the surface of a 15 cm-square glazed tile (AB02E01) in a manner similar to Example 27. Then an aqueous solution containing 1 weight percent of copper acetate monohydrate was applied onto the surface of the tile which was then subjected to irradiation with UV light of a BLB fluorescent lamp to thereby subject copper acetate monohydrate to photoreduction to form a copper deposit whereby a specimen coated with copper-doped titania was obtained. The contact angle with water as measured was less than 3.degree.. As the tile was then tested for the antibacterial function in a manner similar to Example 19, the survival rate of colibacillus was less than 10%. Example 40 Glazed Tile--Photo-Redox Activity Enhancer A coating comprised of titania and silica was formed on the surface of a 15 cm-square glazed tile (AB02E01) in a manner similar to Example 27. Then, the surface of the specimen was doped with platinum in a manner similar to Example 22. The contact angle with water as measured was 0.degree.. The removal rate of methyl mercaptan as measured in a manner similar to Example 20 was 98%. Example 41 Effect of Photoexciting Wavelength After being kept in the dark for 10 days, the #8 specimen of Example 32 and, for the purposes of comparison, the glazed tile (AB02E01) without titania coating were subjected to irradiation with UV light by using a Hg-Xe lamp under the conditions shown in Table 12 and on doing so the variation in response to time of the contact angle with water was measured. TABLE 12 UV Wavelength (nm) 313 365 405 UV Intensity Photon Density (mW/cm.sup.2) (photon/sec/cm.sup.2) 10.6 1.66 .times. 10.sup.16 18 3.31 .times. 10.sup.16 6 1.22 .times. 10.sup.16 The results of measurement were shown in FIGS. 18A-18C wherein the value plotted by white dots represents the contact angle with water of the #8 specimen of Example 32 and the value plotted by black dots indicates the contact angle with water of the glazed tile which was not provided with the titania coating. As will be understood from FIG. 18C, hydrophilification did not occur in the case that a UV light having an energy lower than that of a wavelength of 387 nm corresponding to the bandgap energy of the anatase form of titania (i.e., a UV light having a wavelength longer than 387 nm) was irradiated. In contrast, as will be apparent from FIGS. 18A and 18B, the surface was rendered hydrophilic upon irradiation with UV light having an energy higher than the bandgap energy of anatase. From the foregoing, it was confirmed that hydrophilification of a surface would not occur unless the photocatalyst is photoexcited and that hydrophilification of a surface results from the photocatalytic action of the photocatalyst. Example 42 Physisorption of Water under Photocatalytic Action Powders of the anatase form of titania (made by Nihon Aerosol, P-25) were pressed to form three specimens in the form of a disc of compacted powders. The specimens were subjected respectively to Experiments 1-3, described below, wherein the surface of the specimens was tested and inspected by the Fourier transform infrared spectroscopic analysis (FT-IR) using a Fourier transform infrared spectrometer (FTS-40A). Throughout these experiments, an ultraviolet lamp (UVL-21) having a wavelength of 366 nm was used for UV irradiation. For the purpose of analyzing the infrared absorption spectrum, the following absorption bands are assigned, respectively, to the following information. Sharp absorption band at wavenumber 3690 cm.sup.-1 : stretching of OH bond of chemisorbed water. Broad absorption band at wavenumber 3300 cm.sup.-1 : stretching of OH bond of physisorbed water. Sharp absorption band at wavenumber 1640 cm.sup.-1 : bending of HOH bond of physisorbed water. Absorption bands at wavenumbers 1700 cm.sup.-1, 1547 cm.sup.-1, 1475 cm.sup.-1, 1440 cm.sup.-1, and 1365 cm.sup.-1 : carbonyl groups of contaminants adsorbed onto the specimen surface. Experiment 1 First, the titania disc immediately after press forming was subjected to the infrared spectroscopic analysis. The absorption spectrum of the disc immediately after press forming is shown by the curve #1 in the graphs of FIGS. 19A and 19B. After keeping the titania disc for 17 hours in a dry box containing silica gel as a desiccant, the absorption spectrum was detected which is indicated by the curve #2 in the graphs of FIGS. 19A and 19B. As will be understood upon comparison of the #1 spectrum with the #2 spectrum, infrared absorption at the wavenumber 3690 cm.sup.-1 was drastically decreased in the #2 spectrum, indicating that chemisorbed water has decreased. Similarly, absorption at the wavenumbers 3300 and 1640 cm.sup.-1 was drastically decreased in the #2 spectrum, indicating that physisorbed (physically adsorbed) water has also decreased. It is therefore observed that both chemisorbed water and physisorbed water have decreased by keeping the specimen in dry air for 17 hours. In contrast, infrared absorption at wavenumber 1300-1700 cm.sup.-1 due to presence of the carbonyl groups was increased, suggesting that, during storage of the specimen, compounds containing carbonyl groups were adsorbed onto the specimen surface thereby contaminating the surface. It was impossible to measure the variation in the contact angle with water at the surface of the specimen because of the porous nature of surface of the disc-shaped specimen which was made by press-forming of titania powders. However, it is presumed that the contact angle with water at the surface of a specimen would be increased during storage in dry air if the specimen were made in the form of a thin film of the anatase form of titania. Then, the titania disc in the dry box was subjected to irradiation with UV light for an hour, at a UV intensity of 0.5 mW/cm.sup.2 and the absorption spectrum was detected which is shown in the graphs of FIGS. 19A and 19B by the curve #3. As will be apparent from the #3 spectrum, absorption at wavenumber 3690 cm.sup.-1 was almost revived. Similarly, absorption at wavenumbers 3300 and 1640 cm.sup.-1 substantially restored the initial level. It is therefore observed that, upon UV irradiation, both the amount of chemisorbed water and the amount of physisorbed water are resumed the initial level. It is presumed that, if the specimen were made in the form of a titania thin film, the surface of the thin film would be rendered hydrophilic upon UV irradiation so that the contact angle with water would be decreased. Thereafter, the specimen was placed for 24 hours in a dark room communicated with the ambient air and the absorption spectrum was detected. To avoid various curves being overly complicated, the detected absorption spectrum is shown in the different graphs of FIGS. 20A and 20B by the curve #4. Further, to provide a basis for comparison, the #2 spectrum is reproduced in the graphs of FIGS. 20A and 20B. As shown by the #4 curve, a slight decrease is observed in the absorption at wavenumbers 3690 and 1640 cm.sup.-1. Accordingly, it is concluded that the amount of chemisorbed and physisorbed water slightly decreases as the specimen after UV irradiation is placed in the dark in the presence of moisture in the ambient air. However, absorption at wavenumber 1300-1700 cm.sup.-1 is increased, showing that carbonyl compounds were further adsorbed. It is presumed that, if the specimen were made in the form of a titania thin film, the contact angle with water would be increased in response to contamination. Finally, the titania disc was again subjected to irradiation with UV light in a dark room communicated with the ambient air for an hour, at a UV intensity of 0.5 mW/cm.sup.2 and the absorption spectrum was detected which is shown in the graphs of FIGS. 20A and 20B by the curve #5. As shown in the graphs, no change was observed in the absorption at wavenumber 3690 cm.sup.-1, whereas the absorption at wavenumber 3300 cm.sup.-1 is remarkably increased, with the absorption at wavenumber 1640 cm.sup.-1 being increased. It will therefore be noted that as a result of re-irradiation with UV light, the amount of chemisorbed water remained unchanged but the amount of water was increased. It is presumed that, if the specimen were made in the form of a titania thin film, the contact angle with water would be decreased upon UV irradiation. Experiment 2 First, the titania disc immediately after press forming was subjected to the infrared spectroscopic analysis. The absorption spectrum detected is shown in the graphs of FIGS. 21A and 21B by the curve #1. Then, the titania disc was subjected to irradiation with UV light for one hour, at a UV intensity of 0.5 mW/cm.sup.2 and the absorption spectrum was detected which is shown in the graphs of FIGS. 21A and 21B by the curve #2. The disc was further subjected to irradiation with UV light at the same UV intensity for additional one hour (total 2 hours), further additional one hour (total 3 hours), and further additional 2 hours (total 5 hours) and the absorption spectra detected at the end of irradiation are shown in FIGS. 22A and 22B by the curve #3, #4 and #5, respectively. As will be understood upon comparison of the #1 spectrum with the #2 spectrum, both the amount of chemisorbed water and the amount of physisorbed water were increased as the disc was subjected for the first time to UV irradiation. During the first irradiation, the amount of adherent carbonyl compounds was slightly increased. Presumably, the contact angle with water would be decreased in response to UV irradiation if the specimen were made in the form of a titania thin film. After the disc was subjected to UV irradiation for further one hour (total 2 hours), the amount of chemisorbed water was slightly decreased but the amount of physisorbed water remained unchanged, as shown by the #2 and #3 spectra. The amount of adherent carbonyl compounds was slightly increased. It is considered that the absence of any change in the amount of physisorbed water is due to saturation of the physisorbed water. It is presumed that the contact angle with water would remain unchanged if the specimen were made in the form of a titania thin film. As will be noted from the #4 and #5 spectra, UV irradiation for further one hour (total 3 hours) and for further 2 hours (total 5 hours) resulted in a further slight decrease in the amount of chemisorbed water, with the amount of physisorbed water remained unchanged. The amount of adhered carbonyl compounds was increased. It is considered that the contact angle with water would remain unchanged if the UV irradiation were carried out on a specimen made in the form of a titania thin film. Experiment 3 This experiment is similar to Experiment 1 in many respects and the major difference resides in that the UV intensity was decreased. First, the titania disc immediately after press forming was subjected to the infrared spectroscopic analysis. The detected absorption spectrum is shown by the curve #1 in the graphs of FIGS. 23A and 23B. Then, the disc was placed for 34 hours in a dark room communicated with the ambient air and thereafter the absorption spectrum was detected which is shown by the curve #2 in the graphs of FIGS. 23A and 23B. Then, the titania disc placed in the same dark room was subjected to irradiation with UV light for 2 hours, at a UV intensity of 0.024 mW/cm.sup.2 and the absorption spectrum was detected, the detected spectrum being indicated by the curve #3 in the graphs of FIGS. 23A and 23B. As will be understood from the graphs, both the amount of chemisorbed water and the amount of physisorbed water were decreased as the disc was placed in a dark room in the presence of ambient moisture. As the amount of carbonyl compounds adhered to the specimen was increased, it is presumed that the contact angle with water would be increased if a specimen made in the form of a titania thin film were used. It will be noted that in response to UV irradiation the amount of chemisorbed water was slightly increased and the amount of physisorbed water was increased to again attain to the initial level. During UV irradiation, the amount of adherent carbonyl compounds was slightly increased. It is presumed that the contact angle with water would be increased during UV irradiation if a specimen made in the form of a titania thin film were used. Evaluation of the Test Results To facilitate comparison, the results of Experiments 1-3 are summarized in Table 13 below. Contact Chemisorbed Physisorbed Carbonyl Experiment Angle w/w Water Water Compound Experiment 1 (0.5 mW/cm.sup.2) dark room increased dry air UV irradiated decreased dry air dark room increased ambient air UV irradiated decreased ambient air Experiment 2 (0.5 mW/cm.sup.2) UV irradiated decreased (1 h) UV irradiated unchanged (2 h) UV irradiated unchanged (3 h) UV irradiated unchanged (5 h) Experiment 3 (0.024 mW/ cm.sup.2) dark room increased ambient air UV irradiated decreased ambient air decreased decreased increased almost restored slightly decreased unchanged restored decreased slightly decreased increased increased slightly increased slightly decreased slightly decreased slightly increased unchanged slightly increased slightly increased increased unchanged increased decreased decreased increased slightly increased increased increased unchanged unchanged decreased As will be best understood from Table 13, the amount of physisorbed water increases in good response to UV irradiation. In this regard, it is considered that, as illustrated in the upper part of FIG. 24, in the crystal face of a crystal of titania forming a titania coating 30, a terminal OH group 32 is bonded to each titanium atom, with a bridging OH group 34 being bonded to a pair of adjacent titanium atoms, these OH groups 32 and 34 forming a layer of chemisorbed water. It is considered that, upon irradiation with UV light in the presence of ambient moisture, molecules of water in the ambient air are physically adsorbed by way of hydrogen bond 36 onto the hydrogen atoms of the terminal and bridging OH groups to thereby form a layer of physisorbed water 38, as illustrated in the lower part of FIG. 24. As the amount of physisorbed water increases in good response to UV irradiation as described before, Example 42 demonstrates that formation of a layer of physisorbed water 38 is induced by the photocatalytic action of titania. It is believed that because of the presence of the layer of physisorbed water 38 the surface of titania surface is rendered hydrophilic. In contrast, the amount of carbonyl compounds adhered to the surface appears to increase with increasing duration of contact with ambient air. It is considered that upon photoexcitation of the photocatalyst the water-wettability of the surface is increased regardless of increasing amount of adherent carbonyl compounds. Example 43 Plastic Plate Coated by Photocatalyst-Containing Silicone A titania-containing coating composition similar to that of Example 18 was applied on a polyethyleneterephthalate (PET) film (Fuji Xerox, monochromatic PPC film for OHP, JF-001) and was cured at a temperature of 110.degree. C. to obtain #1 specimen coated with titania-containing silicone. Further, an aqueous polyester paint (made by Takamatsu Resin, A-124S) was applied on another PET film (JF-001) and was cured at 110.degree. C. to form a primer coating. A titania-containing coating composition similar to that of Example 18 was then applied on the primer coating and was cured at a temperature of 110.degree. C. to obtain #2 specimen. Also, a titania-containing coating composition similar to that of Example 18 was applied on a polycarbonate (PC) plate and was cured at a temperature of 110.degree. C. to obtain #3 specimen. Furthermore, an aqueous polyester paint (A-124S) was applied on another polycarbonate plate, followed by curing at a temperature of 110.degree. C. to form a primer coating, and a titania-containing coating composition similar to that of Example 18 was thereafter applied thereon followed by curing at a temperature of 110.degree. C. to obtain #4 specimen. The #1-#4 specimens as well as the PET film (JF-001) and polycarbonate plate as such were subjected to irradiation with UV light, at a UV intensity of 0.6 mW/cm.sup.2 by using a BLB fluorescent lamp and on doing so the variation in response to time of the contact angle with water of the specimen surface was measured. The results are shown in Table 14. TABLE 14 Before Specimen #1 2.degree. #2 2.degree. #3 3.degree. #4 2.degree. PET 1 day 2 days 3 days 10 days Irradiat. later later later later 71.degree. 44.degree. 32.degree. 7.degree. 73.degree. 35.degree. 16.degree. 3.degree. 66.degree. 55.degree. 27.degree. 9.degree. 65.degree. 53.degree. 36.degree. 18.degree. 70.degree. 72.degree. 74.degree. 73.degree. 90.degree. 86.degree. 88.degree. 87.degree. 60.degree. PC 89.degree. As will be apparent from Table 14, the surface of the specimens under question was hydrophilified as UV irradiation was continued and about 3 days later the surface is rendered superhydrophilic. As described hereinbefore with reference to Example 14, it is considered that this is due to the fact that the organic groups bonded to the silicon atoms of the silicone molecules of the titania-containing silicone layer were substituted with the hydroxyl groups under the photocatalytic action caused by photoexcitation. As is well-known, a UV intensity of 0.6 mW/cm.sup.2 is roughly equal to the intensity of the UV light contained in the sunlight impinging upon the earth's surface. It will be noted, accordingly, that superhydrophilification can be achieved simply by exposing the titania-containing silicone coating to the sunlight. Example 44 Weathering Test of Photocatalyst-Containing Silicone The #1 specimen (aluminum substrate coated with silicone) and the #2 specimen (aluminum substrate coated with titania-containing silicone) of Example 13 were subjected to a weathering test by using a weathering testing machine (made by Suga Testing Instruments, Model "WEL-SUN-HC") while irradiating a light from a carbon arc lamp and spraying rain for 12 minutes per hour and at a temperature of 40.degree.C. The weather resistivity was assessed by the glossiness retention rate (percentage of the glossiness after testing to the initial glossiness). The results are shown in Table 15. TABLE 15 Specimen #1 #2 500 hrs 91 99 1000 hrs 95 100 3000 hrs 90 98 As will be apparent from Table 15, the glossiness retention rate remained roughly the same regardless of the presence or absence of titania. This indicates that the siloxane bonds forming the main chain of the silicone molecule were not broken by the photocatalytic action of titania. It is therefore considered that the weather resistivity of silicone is not affected even after the organic groups bonded to the silicon atoms of the silicone molecules are substituted with the hydroxyl groups. While the present invention has been described herein with reference to the specific embodiments thereof, it is contemplated that the invention is not limited thereby and various modifications and alterations may be made therein without departing from the scope of the invention. Furthermore, the present invention may be applied for various purposes and fields other than the aforesaid. For example, a superhydrophilified surface may be utilized to prevent air bubbles from adhering to an underwater surface. Also, the superhydrophilified surface may be used to form and maintain a uniform film of water. Moreover, in view of an excellent affinity for vital tissues and organs, the superhydrophilic photocatalytic coating may be utilized in the medical fields such as contact lens, artificial organs, catheters, and anti-thrombotic materials. (4 United States Patent Boire , of 4) 6,680,135 et al. January 20, 2004 Substrate with a photocatalytic coating Abstract The subject of the invention is a glass-, ceramic- or vitroceramic-based substrate (1) provided on at least part of at least one of its faces with a coating (3) with a photocatalytic property containing at least partially crystalline titanium oxide. It also relates to the applications of such a substrate and to its method of preparation. Inventors: Boire; Philippe (Paris, FR); Talpaert; Xavier (Paris, FR) Assignee: Saint-Gobain Glass France (Paris, FR) Appl. No.: 079484 Filed: February 22, 2002 Foreign Application Priority Data Sep 15, 1996[FR] 95 10839 Current U.S. Class: 428/702; 428/336; 428/428; 428/432; 428/450; 428/469; 428/496; 428/523; 428/632; 428/687 Intern'l Class: B32B 015/04; B32B 017/06; G03C 001/725; G03C 001/76 Field of 428/432,433,434,632,633,660,687,472,472.1,701,702,937,428,450,469,699,332,336 430/270.1,496,271.1,523,272.1,273.1 Search: References Cited [Referenced By] U.S. Patent Documents 3630796 Dec., 1971 Yokozawa et al. 156/17. 4017661 Apr., 1977 Gillery 428/412. 4112142 Sep., 1978 Schroder et al. 427/166. 4238276 Dec., 1980 Kinugawa et al. 156/634. 4485146 Nov., 1984 Mizuhashi et al. 4544470 Oct., 1985 Hetrick 4888038 Dec., 1989 Herrington et al. 65/114. 4892712 Jan., 1990 Robertson et al. 422/186. 4898789 Feb., 1990 Finley. 4997576 Mar., 1991 Heller et al. 5028568 Jul., 1991 Anderson et al. 501/12. 5032241 Jul., 1991 Robertson et al. 204/157. 5035784 Jul., 1991 Anderson et al. 204/158. 5165972 Nov., 1992 Porter. 5304394 Apr., 1994 Sauvinet et al. 5308805 May., 1994 Baker et al. 5342676 Aug., 1994 Zagdoun. 5348805 Sep., 1994 Zagdoun et al. 5368892 Nov., 1994 Berquier 427/299. 5389427 Feb., 1995 Berquier 428/210. 5478783 Dec., 1995 Higby et al. 5505989 Apr., 1996 Jenkinson 427/166. 5514454 May., 1996 Boire et al. 428/216. 5525406 Jun., 1996 Goodman et al. 428/216. 5547823 Aug., 1996 Murasawa et al. 430/531. 5580364 Dec., 1996 Goodman et al. 65/60. 5584169 Dec., 1996 Ikehara 204/248. 427/166. 501/71. 501/27. 502/242. 5616532 Apr., 1997 Heller et al. 502/242. 5618579 Apr., 1997 Boire et al. 427/166. 5698262 Dec., 1997 Soubeyrand et al. 427/255. 5735922 Apr., 1998 Woodward et al. 65/104. 5745291 Apr., 1998 Jenkinson 5749931 May., 1998 Goodman et al. 65/60. 5751484 May., 1998 Goodman et al. 359/512. 5755845 May., 1998 Woodward et al. 65/102. 5764415 Jun., 1998 Nelson et al. 359/584. 5773086 Jun., 1998 McCurdy et al. 472/255. 5811192 Sep., 1998 Takahama et al. 428/432. 5853866 Dec., 1998 Watanabe et al. 428/312. 5869187 Feb., 1999 Nakamura et al. 428/428. 5939201 Aug., 1999 Boire et al. 428/432. 5961843 Oct., 1999 Hayakawa et al. 210/748. 6001462 Dec., 1999 Purvis et al. 428/215. 6013372 Jan., 2000 Hayakawa et al. 428/411. 6027766 Feb., 2000 Greenberg et al. 427/226. 6027797 Feb., 2000 Watanabe et al. 428/312. 6037289 Mar., 2000 Chopin et al. 6045896 Apr., 2000 Boire et al. 428/216. 6054227 Apr., 2000 Greenberg et al. 428/701. 6068914 May., 2000 Boire et al. 428/216. 6090489 Jul., 2000 Hayakawa et al. 428/409. 6103360 Aug., 2000 Caldwell et al. 428/323. 6103363 Aug., 2000 Boire et al. 428/325. 6106955 Aug., 2000 Ogawa et al. 428/469. 6210779 Apr., 2001 Watanabe et al. 6228480 May., 2001 Kimura et al. 428/328. 6238738 May., 2001 McCurdy 427/255. 6294246 Sep., 2001 Watanabe et al. 6294247 Sep., 2001 Watanabe et al. 6322881 Nov., 2001 Boire et al. 359/586. 502/2. 432/216. 6326079 Dec., 2001 Philippe et al. 428/325. 6387844 May., 2002 Fujishima et al. 502/350. Foreign Patent Documents 0 071 865 Feb., 1983 EP. 0 348 185 Dec., 1989 EP. 489 621 Jun., 1992 EP. 0 544 577 Jun., 1993 EP. 0 581 216 Feb., 1994 EP. A 581 216 Feb., 1994 EP. 590 477 Apr., 1994 EP. 633 064 Jan., 1995 EP. 636 702 Feb., 1995 EP. 650 938 May., 1995 EP. 675 086 Oct., 1995 EP. 737 513 Oct., 1996 EP. 816 466 Jan., 1998 EP. 2031756 Apr., 1980 GB. 2 320 499 Jun., 1998 GB. 2 320503 Jun., 1998 GB. 2 324098 Oct., 1998 GB. 2 355 273 Apr., 2001 GB. 5-3149281 Dec., 1978 JP. 91042/1986 May., 1986 JP. 63-100042 May., 1988 JP. 5-302173 Nov., 1993 JP. 7-99425 Mar., 1995 JP. 7-117600 Apr., 1995 JP. 7-182019 Jun., 1995 JP. 7-182020 Jun., 1995 JP. 7-205019 Jul., 1995 JP. 8-119673 May., 1996 JP. 8-164334 Jun., 1996 JP. 8-313705 Nov., 1996 JP. 9-173783 Jul., 1997 JP. 9-227157 Sep., 1997 JP. 9-227158 Sep., 1997 JP. 9-235140 Sep., 1997 JP. 9-241037 Feb., 1999 JP. WO 95/15816 Jun., 1995 WO. WO 96/13327 May., 1996 WO. WO 96/29375 Sep., 1996 WO. WO 97/07069 Feb., 1997 WO. WO 97/10186 Mar., 1997 WO. WO 98/06510 Feb., 1998 WO. WO 98/06675 Feb., 1998 WO. WO 00/75087 Dec., 2000 WO. WO 01/10790 Feb., 2001 WO. WO 01/72652 Oct., 2001 WO. Other References Opposition filed by Glaverbel (no date). Opposition filed by Pilkington PLC (no date). Opposition filed by Toto LTD. (no date). Opposition filed by Sternagel, Fleischer, Godemeyer, Aug. 2002. Pt-TiO.sub.2 Thin Films on Glass Substrates as Efficient Photocatalysts, Journal of Materials Science, 24 (1989), pp. 243-246. (no month). Preparation of TiO.sub.2 Fine Particles Supported on Silica Gel as Photocatalyst, Yasumori et al., Journal of the Ceramic Society of Japan, 102 (1994). (Aug.). Photocatalytic Properties of TiO.sub.2, Wold, Chem. Mater., 1993, 5, pp. 280-283. (no month). Kristallstruktur und Optische Eigenshaften von Dunnen Organogenen Titanoxyd-Schichten Auf Glasunterlagen [Crystal Structure and Optical Properties of Thin Organogenic Titanium Oxide Layers on Glass Substrates], Bach et al., Thin Solid Films I (1967/68), pp. 255-276, and English translation (no month). The Effect of Substrate Temperature on the Properties of Sputtered Titanium Oxide Films, Meng et al., Applied Surface Science 65/66 (1993) pp. 235-239. (no month). Sol-Gel-Derived TiO.sub.2 Film Semiconductor Electrode for Photocleavage of Water, Yoko et al., J. Electrochem. Soc., vol. 138(8), Aug. 1991, pp. 2279-2284. Dip-Coating of TiO.sub.2 Films Using a Sol Derived from Ti(O-iPr).sub.4 -diethamolamine-H.sub.2 O-i-PrOH System, Takahashi et al., Journal of Materials Science 23 (1988, pp. 2259-2266. (no month). A Structural Investigation of Titanium Dioxide Photocatalysts, Bickley, et al., Journal of Solid State Chemistry 92, 1991, pp. 178-190. (no month). Highly Transparent and Photoactive TiO2 Thin Film Coated on Glass Substrate, Fukayama et al., Abstract 735, 187.sup.th Electrochemical Society Meeting, Mar. 29, 1995. EP 684,075, Wantanabe et al., Jun. 15, 1995 and the front page of WO 95/15816, Watanabe et al., Jun. 15, 1995, which is an equivalent of EP 684,075 and contains an English abstract. Oxide Layers Deposited from Organic Solutions, H. Schroeder in Physics of Thin Films: Advances in Research and Development pp. 105-112, vol. 5, 1969 Academic Press. (no month). JP 267476/94, Fujishima et al., (no date) publication date not listed, and Derwent WPIndex abstract; corresponds to EP 737 513A, (submitted herewith under Tab 17); WO 96/13327 (of record; published on May 9, 1996); and U.S. 6,387,844 (of record). JPA 63-5301 Matsushita Electric Works (assignee), Jan. 11, 1988, and Derwent WPIndex abstract. JPA 63-5304, Matsushita Electric Works (assignee), Jan. 11, 1988, and Derwent WPIndex abstract. JPA 7-222928, Chikuni et al., Aug. 22, 1995, and Derwent WPIndex abstract; equivalent of WO 95/15816 (submitted herewith)) and U.S. 5,853,866 (of record) 6,210,779 (submitted hereiwth), 6,294,246 (submitted herewith), and 6,294,247 (submitted herewith). JPA 1-218635, Hitachi LTD (assignee), Feb. 29, 1988, and Derwent WPIindex abstract. JPA 7-111104, Fujishima et al., Apr. 25, 1995, and Derwent WPIndex abstract. Preparation of Transparent TiO.sub.2 Thin Film Photocatalyst and Its Photocatalytic Activity, Negishi et al., Chemistry Letters 1995, No. 9, pp. 841-842. (Sep.). JP-A-5 253 544, Toto LTD (assignee), Oct. 5, 1995, and Derwent WPIndex abstract. JP-A-6-65012, Agency of Ind. Sci. & Technology (assignee), Mar. 8, 1994, and Derwent WPIndex abstract. Electrical and Electrochemical Properties of TiO.sub.2 films Grown by Organometallic Chemical Vapour Deposition, Takahashi et al., J. Chem. Soc., Faraday Trans 1, 1982, 78, pp. 2563-2571. (no month). Apr. 3, 2000 Letter from Mrs.Colette Ward, Patent Litigation Enquiries, British Library. Table showing values of roughness (Root Mean Square) of various coated glass samples available tot the public at the priority date of EP 850 204. (no date). Masanari Takahashi et al., "Pt-TiO.sub.2 Thin Films on Glass Substrates as Efficient Photocatalysts," Journal of Materials Science, 24 (1989) pp. 243-246, (Jan.). S. Fukuyama et al., Highly Transparent and Photoactive TiO.sub.2 Thin Film Coated on Glass Substrate, Abstract No. 735, pp. 1102-1103, 187.sup.th Electrochemical Society Meeting, Reno, NV, May 21-26, 1995, and attached facsimile transmission from The British Library indicating that the date of availability for public use was Mar. 29, 1995. D.R. Uhlmann, "Glass: Science and Technology," vol. 2, Processing I, pp. 253-283, 1984, (no month). GlassFAQs, GlassFACTS.com, p. 1-5. (no date). JP 63-100042; May 1988. Abstract Only, Derwent AN 88-158990, Glass Article Difficult Soil Coating Thin Titanium DI Oxide Coating Contain Platinum@ Rhodium Palladium@. Chemical Abstracts,, 116: 89812a, Sokolov, et al., "Selective Activation of Smooth Surfaces of Glass and Ceramic Articles Before Chemical Metalization," Abstract Only. SU 166304; Jul. 1991. International Search Report for PCT/FR96/01421, Jan. 1997. International Preliminary Examination Report for PCT/FR96/01421, May 1997. Robert J. Good et al., The Modern Theory of Contact Angles and the Hydrogen Bond Components of Surface Energies, in Modern Approaches to Wettability, Theory and Applications, pp 1-3, Schrader et al., Eds. (no date). H.H. Dunken, Glass Surfaces, Treatise on Materials Science and Technology, vol. 22, pp. 1-75, 1982. (no month). Toshiya Watanabe et al., Photocatalytic Activity of TiO2 Thin Film under Room Light, in Photocatalytic Purification and Treatment of Water and Air, pp. 747-751, 1993, Ollis et al., Eds. (no month). The Japan Times, May 21, 2002, Tuesday, Race for Technology, pp. 2-5, from LexisNexis. P. Chartier, Verre, Glass Institute, vol. 3, No. 3--Jun. 1997, pp. 5-12, French document with English Translation. U.S. patent application Ser. No. 09/486,719, filed Aug. 2, 2000, pending. U.S. patent application Ser. No. 09/923,353, filed Aug. 8, 2001, pending. U.S. patent application Ser. No. 09/940,499, filed Aug. 29. 2001, pending. U.S. patent application Ser. No. 10/000,503, filed Dec. 4, 2001, pending. U.S. patent application Ser. No. 10/079,484, filed Feb. 22, 2002, pending. U.S. patent application Ser. No. 10/079,483, filed Feb. 22, 2002, pending. U.S. patent application Ser. No. 10/079,533, filed Feb. 22, 2002, pending. U.S. patent application Ser. No. 09/719,153, filed Mar. 16, 2001, pending. U.S. patent application Ser. No. 10/116,164, filed Apr. 5, 2002, pending. U.S. patent application Ser. No. 10/220,268, filed Sep. 6, 2002, pending. Primary Examiner: LaVilla; Michael Attorney, Agent or Firm: Oblon, Spivak, McClelland, Maier & Neustadt, P.C. Parent Case Text This application is a Continuation of application Ser. No. 10/000,503, filed on Dec. 4, 2001, now abandoned, which is a continuation of application Ser. No. 09/615,910, filed Jul. 13, 2000, now U.S. Pat. No. 6,326,079, which is a continuation of application Ser. No. 09/029,855, filed on May 28, 1998, now U.S. Pat. No. 6,103,363, which was originally filed as PCT/FR96/01421 on Sep. 13, 1996. Claims What is claimed is: 1. A self-cleaning article of manufacture, comprising: a glass-, ceramic-, or vitroceramic-based substrate having at least one surface over which organic contaminants are expected to accumulate, a photocatalytically-activated self-cleaning coating of a photocatalytically-activated self-cleaning and at least partially crystalline titanium oxide deposited on said at least one surface by sol-gel or liquid spray pyrolysis, and a barrier layer with respect to alkali metals between said at least one surface and the coating which comprises a member selected from the group consisting of silicon nitride, silicon oxynitride, silicon oxycarbide, Al.sub.2 O.sub.3 :F, and aluminum nitride. 2. The self-cleaning article of manufacture of claim 1, wherein the crystalline form of the titanium dioxide is selected from the group consisting of anatase, rutile, and combinations of anatase and rutile. 3. The self-cleaning article of manufacture of claim 1, wherein the self-cleaning coating has a thickness between 5 nm and 1 micron. 4. The self-cleaning article of manufacture of claim 1, wherein the photocatalytically-activated self-cleaning coating is photocatalytically-activated to be self-cleaning upon irradiation with ultraviolet radiation. 5. The self-cleaning article of manufacture of claim 1, which further comprises one or more ultraviolet lamps. 6. The self-cleaning article of manufacture of claim 1, wherein the barrier layer comprises silicon nitride. 7. The self-cleaning article of manufacture of claim 1, wherein the barrier layer comprises silicon oxynitride. 8. The self-cleaning article of manufacture of claim 1, wherein the barrier layer comprises silicon oxycarbide. 9. The self-cleaning article of manufacture of claim 1, wherein the barrier layer comprises Al.sub.2 O.sub.3 :F. 10. The self-cleaning article of manufacture of claim 1, wherein the barrier layer comprises aluminum nitride. Description The invention relates to glass-, ceramic- or vitroceramic-based substrates, more particularly made of glass, in particular transparent substrates, which are furnished with coatings with photocatalytic properties, for the purpose of manufacturing glazing for various applications, such as utilitarian glazing or glazing for vehicles or for buildings. There is an increasing search to functionalize glazing by depositing at the surface thereof thin layers intended to confer thereon a specific property according to the targeted application. Thus, there exist layers with an optical function, such as so-called anti-glare layers composed of a stack of layers alternatively with high or low refractive indices. For an anti-static function or a heating function of the anti-icer type, it is also possible to provide electrically conducting thin layers, for example based on metal or doped metal oxide. For an anti-solar or low-emissivity thermal function for example, thin layers made of metal of the silver type or based on metal oxide or nitride may be used. To obtain a "rain-repellent" effect, it is possible to provide layers with a hydrophobic nature, for example based on fluorinated organosilane and the like. However, there still exists a need for a substrate, particularly a glazing, which could be described as "dirt-repellent", that is to say targeted at the permanence over time of the appearance and surface properties, and which makes it possible in particular to render cleaning less frequent and/or to improve the visibility, by succeeding in removing, as they are formed, the dirty marks which are gradually deposited at the surface of a substrate, in particular dirty marks of organic origin, such as finger marks or volatile organic products present in the atmosphere, or even dirty marks of condensation type. In point of fact, it is known that there exist certain semiconductive materials based on metal oxides which are capable, under the effect of radiation of appropriate wavelength, of initiating radical reactions which cause the oxidation of organic products; they are generally referred to as "photocatalytic" or alternatively "photoreactive" materials. The aim of the invention is then to develop photocatalytic coatings on a substrate which exhibit a marked "dirt-repellent" effect with respect to the substrate and which can be manufactured industrially. The object of the invention is a glass-, ceramic- or vitroceramic-based substrate, in particular made of glass and transparent, provided on at least part of at least one of its faces with a coating with a photocatalytic property containing at least partially crystalline titanium oxide. The titanium oxide is preferably crystallized "in situ" during the formation of the coating on the substrate. Titanium oxide is in fact one of the semiconductors which, under the effect of light in the visible or ultraviolet range, degrade organic products which are deposited at their surface. The choice of titanium oxide to manufacture a glazing with a "dirt-repellent" effect is thus particularly indicated, all the more so since this oxide exhibits good mechanical strength and good chemical resistance: for long-term effectiveness, it is obviously important for the coating to retain its integrity, even if it is directly exposed to numerous attacks, in particular during the fitting of the glazing on a building site (building) or on a production line (vehicle) which involves repeated handlings by mechanical or pneumatic prehension means, and also once the glazing is in place, with risks of abrasion (windscreen wipers, abrasive rag) and of contact with aggressive chemicals (atmospheric pollutants of SO.sub.2 type, cleaning product, and the like). The choice has fallen, in addition, on a titanium oxide which is at least partially crystalline because it has been shown that it had a much better performance in terms of photocatalytic property than amorphous titanium oxide. It is preferably crystallized in the anatase form, in the rutile form or in the form of a mixture of anatase and rutile, with a degree of crystallization of at least 25%, in particular of approximately 30 to 80%, in particular close to the surface (the property being rather a surface property). (Degree of crystallization is understood to mean the amount by weight of crystalline TiO.sub.2 with respect to the total amount by weight of TiO.sub.2 in the coating). It has also been possible to observe, in particular in the case of crystallization in anatase form, that the orientation of the TiO.sub.2 crystals growing on the substrate had an effect on the photocatalytic behaviour of the oxide: there exists a favoured orientation (1, 1, 0) which markedly promotes photocatalysis. The coating is advantageously manufactured so that the crystalline titanium oxide which it contains is in the form of "crystallites", at least close to the surface, that is to say of monocrystals, having an average size of between 0.5 and 100 nm, preferably 1 to 50 nm, in particular 10 to 40 nm, more particularly between 20 and 30 nm. It is in fact in this size range that titanium oxide appears to have an optimum photocatalytic effect, probably because the crystallites of this size develop a high active surface area. As will be seen in more detail subsequently, it is possible to obtain the coating based on titanium oxide in many of ways: by decomposition of titanium precursors (pyrolysis techniques: liquid pyrolysis, powder pyrolysis, pyrolysis in the vapour phase, known as CVD (Chemical Vapour Deposition), or techniques associated with the sol-gel: dipping, cell coating, and the like), by a vacuum technique (reactive or non-reactive cathodic sputtering). The coating can also contain, in addition to the crystalline titanium oxide, at least one other type of inorganic material, in particular in the form of an amorphous or partially crystalline oxide, for example a silicon oxide (or mixture of oxides), titanium oxide, tin oxide, zirconium oxide or aluminium oxide. This inorganic material can also participate in the photocatalytic effect of the crystalline titanium oxide, by itself exhibiting to a certain extent a photocatalytic effect, even a weak effect compared with that of crystalline TiO.sub.2, which is the case with tin oxide or amorphous titanium oxide. A layer of "mixed" oxide thus combining at least partially crystalline titanium oxide with at least one other oxide can be advantageous from an optical viewpoint, very particularly if the other oxide or oxides are chosen with a lower index than that of TiO.sub.2 : by lowering the "overall" refractive index of the coating, it is possible to vary the light reflection of the substrate provided with the coating, in particular to lower this reflection. This is the case if, for example, a layer made of TiO.sub.2 /Al.sub.2 O.sub.3, a method for the preparation of which is described in Patent EP-0,465,309, or made of TiO.sub.2 /SiO.sub.2 is chosen. It is necessary, of course, for the coating to contain however a TiO.sub.2 content which is sufficient to maintain a significant photocatalytic activity. It is thus considered that it is preferable for the coating to contain at least 40% by weight, in particular at least 50% by weight, of TiO.sub.2 with respect to the total weight of oxide(s) in the coating. It is also possible to choose to superimpose, with the coating according to the invention, a grafted oleophobic and/or hydrophobic layer which is stable or resistant to photocatalysis, for example based on the fluorinated organosilane described in Patents U.S. Pat. No. 5,368,892 and U.S. Pat. No. 5,389,427 and on the perfluoroalkylsilane described in Patent Application FR-94/08734 of Jul. 13, 1994, published under the number FR-2,722,493 and corresponding to European Patent EP-0,692,463, in particular of formula: CF.sub.3 -(CF.sub.2).sub.n -(CH2).sub.m -SiX3 in which n is from 0 to 12, m is from 2 to 5 and X is a hydrolysable group. To amplify the photocatalytic effect of the titanium oxide of the coating according to the invention, it is possible first of all to increase the absorption band of the coating, by incorporating other particles in the coating, in particular metal particles or particles based on cadmium, tin, tungsten, zinc, cerium or zirconium. It is also possible to increase the number of charge carriers by doping the crystal lattice of the titanium oxide by inserting therein at least one of the following metal elements: niobium, tantalum, iron, bismuth, cobalt, nickel, copper, ruthenium, cerium or molybdenum. This doping can also be carried out by surface doping only of the titanium oxide or of the combined coating, surface doping carried out by covering at least part of the coating with a layer of metal oxides or salts, the metal being chosen from iron, copper, ruthenium, cerium, molybdenum, vanadium and bismuth. Finally, the photocatalytic phenomenon can be accentuated by increasing the yield and/or the kinetics of the photocatalytic reactions, by covering the titanium oxide, or at least part of the coating which incorporates it, with a noble metal in the form of a thin layer of the platinum, rhodium, silver or palladium type. Such a catalyst, for example deposited by a vacuum technique, in fact makes it possible to increase the number and/or the lifetime of the radical entities created by the titanium oxide and thus to promote the chain reactions leading to the degradation of organic products. In an entirely surprising way, the coating exhibits in fact not one property but two, as soon as it is exposed to appropriate radiation, as in the visible and/or ultraviolet field, such as sunlight: by the presence of photocatalytic titanium oxide, as already seen, it promotes the gradual disappearance, as they are accumulated, of dirty marks of organic origin, their degradation being caused by a radical oxidation process. Inorganic dirty marks are not, themselves, degraded by this process: they therefore remain on the surface and, except for a degree of crystallization, they are in part easily removed since they no longer have any reason to adhere to the surface, the binding organic agents being degraded by photocatalysis. However, the coating of the invention, which is permanently self-cleaning, also preferably exhibits an external surface with a pronounced hydrophilic and/or oleophilic nature which results in three very advantageous effects: a hydrophilic nature makes possible complete wetting of the water which can be deposited on the coating. When a water condensation phenomenon takes place, instead of a deposit of water droplets in the form of condensation which hampers visibility, there is in fact a continuous thin film of water which is formed on the surface of the coating and which is entirely transparent. This "anti-condensation" effect is in particular demonstrated by the measurement of a contact angle with water of less than 5.degree. after exposure to light, and after running of water, in particular of rain, over a surface which has not been treated with a photocatalytic layer, many drops of rainwater remain stuck to the surface and leave, once evaporated, unattractive and troublesome marks, mainly of inorganic origin. Indeed, a surface exposed to the surrounding air is rapidly covered by a layer of dirty marks which limits the wetting thereof by water. These dirty marks are in addition to the other dirty marks, in particular inorganic marks (crystallizations and the like), contributed by the atmosphere in which the glazing bathes. In the case of a photoreactive surface, these inorganic dirty marks are not directly degraded by photocatalysis. In fact, they are in very large part removed by virtue of the hydrophilic nature induced by the photocatalytic activity. This hydrophilic nature indeed causes complete spreading of the drops of rain. Evaporation marks are therefore no longer present. Moreover, the other inorganic dirty marks present on the surface are washed, or redissolved in the case of crystallization, by the water film and are thus in large part removed. An "inorganic dirt-repellent" effect is obtained, induced in particular by rain, in conjunction with a hydrophilic nature, the coating can also exhibit an oleophilic nature which makes possible the "wetting" of the organic dirty marks which, as with water, then tend to be deposited on the coating in the form of a continuous film which is less visible than highly localized "stains". An "organic dirt-repellent" effect is thus obtained which operates in two ways: as soon as it is deposited on the coating, the dirty mark is already not very visible. Subsequently, it gradually disappears by radical degradation initiated by photocatalysis. The coating can be chosen with a more or less smooth surface. A degree of roughness can indeed be advantageous: it makes it possible to develop a greater active photocatalytic surface area and thus induces a greater photocatalytic activity, it has a direct effect on the wetting. The roughness in fact enhances the wetting properties. A smooth hydophilic surface will be even more hydrophilic once rendered rough. "Roughness" is understood to mean, in this instance, both the surface roughness and the roughness induced by a porosity of the layer in at least a portion of its thickness. The above effects will be all the more marked when the coating is porous and rough, resulting in a superhydrophilic effect for rough photoreactive surfaces. However, when exaggerated, the roughness can be penalizing by promoting incrustation or accumulation of dirty marks and/or by bringing about the appearance of an optically unacceptable level of fuzziness. It has thus proved to be advantageous to adapt the method for deposition of TiO.sub.2 -based coatings so that they exhibit a roughness of approximately 2 to 20 nm, preferably of 5 to 15 nm, this roughness being evaluated by atomic force microscopy, by measurement of the value of the root mean square or RMS over a surface area of 1 square micrometre. With such roughnesses, the coatings exhibit a hydrophilic nature which is reflected by a contact angle with water which can be less than 1.degree.. It has also been found that it is advantageous to promote a degree of porosity in the thickness of the coating. Thus, if the coating consists only of TiO.sub.2, it preferably exhibits a porosity of the order of 65 to 99%, in particular of 70 to 90%, the porosity being defined in this instance indirectly by the percentage of the theoretical relative density of TiO.sub.2, which is approximately 3.8. One means for promoting such a porosity comprises, for example, the deposition of the coating by a technique of the sol-gel type involving the decomposition of materials of organometallic type: an organic polymer of polyethylene glycol PEG type can then be introduced into the solution, in addition to the organometallic precursor(s): on curing the layer by heating, the PEG is burnt off, which brings about or accentuates a degree of porosity in the thickness of the layer. The thickness of the coating according to the invention is variable; it is preferably between 5 nm and 1 micron, in particular between 5 and 100 nm, in particular between 10 and 80 nm, or between 20 and 50 nm. In fact, the choice of the thickness can depend on various parameters, in particular on the targeted application of the substrate of the glazing type or alternatively on the size of the TiO.sub.2 crystallites in the coating or on the presence of a high proportion of alkali metals in the substrate. It is possible to arrange, between the substrate and the coating according to the invention, one or a number of other thin layers with a different or complementary function to that of the coating. It can concern, in particular, layers with an anti-static, thermal or optical function or promoting the crystalline growth of TiO.sub.2 in the anatase or rutile form or of layers forming a barrier to the migration of certain elements originating from the substrate, in particular forming a barrier to alkali metals and very particularly to sodium ions when the substrate is made of glass. It is also possible to envisage a stack of alternating "anti-glare" layers of thin layers with high and low indices, the coating according to the invention constituting the final layer of the stack. In this case, it is preferable for the coating to have a relatively low refractive index, which is the case when it is composed of a mixed oxide of titanium and of silicon. The layer with an anti-static and/or thermal function (heating by providing it with power leads, low-emissive, anti-solar, and the like) can in particular be chosen based on a conductive material of the metal type, such as silver, or of the doped metal oxide type, such as indium oxide doped with tin ITO, tin oxide doped with a halogen of the fluorine type SnO.sub.2: F or with antimony SnO.sub.2 :Sb or zinc oxide doped with indium ZnO:In, with fluorine ZnO:F, with aluminium ZnO:Al or with tin ZnO:Sn. It can also concern metal oxides which are stoichiometrically deficient in oxygen, such as SnO.sub.2-x or ZnO.sub.2-x with .times.<2. The layer with an anti-static function preferably has a surface resistance value of 20 to 1000 ohms.square. Provision can be made for furnishing it with power leads in order to polarize it (feeding voltages for example of between 5 and 100 V). This controlled polarization makes it possible in particular to control the deposition of dust with a size of the order of a millimetre capable of being deposited on the coating, in particular dry dust which adheres only by an electrostatic effect: by suddenly reversing the polarization of the layer, this dust is "ejected". The thin layer with an optical function can be chosen in order to decrease the light reflection and/or to render more neutral the colour in reflection of the substrate. In this case, it preferably exhibits a refractive index intermediate between that of the coating and that of the substrate and an appropriate optical thickness and can be composed of an oxide or of a mixture of oxides of the aluminium oxide Al.sub.2 O.sub.3, tin oxide SnO.sub.2, indium oxide In.sub.2 O.sub.3 or silicon oxycarbide or oxynitride type. In order to obtain maximum attenuation of the colour in reflection, it is preferable for this thin layer to exhibit a refractive index close to the square root of the product of the squares of the refractive indices of the two materials which frame it, that is to say the substrate and the coating according to the invention. In the same way, it is advantageous to choose its optical thickness (that is to say the product of its geometric thickness and of its refractive index) similar to lambda/4, lambda being approximately the average wavelength in the visible, in particular from approximately 500 to 550 nm. The thin layer with a barrier function with respect to alkali metals can be in particular chosen based on silicon oxide, nitride, oxynitride or oxycarbide, made of aluminium oxide containing fluorine Al.sub.2 O.sub.3 :F or alternatively made of aluminium nitride. In fact, it has proved to be useful when the substrate is made of glass, because the migration of sodium ions into the coating according to the invention can, under certain conditions, detrimentally affect the photocatalytic properties thereof. The nature of the substrate or of the sublayer furthermore has an additional advantage: it can promote the crystallization of the photocatalytic layer which is deposited, in particular in the case of CVD deposition. Thus, during deposition of TiO.sub.2 by CVD, a crystalline SnO.sub.2 :F sublayer promotes the growth of TiO.sub.2 mostly in the rutile form, in particular for deposition temperatures of the order of 400.degree. to 500.degree. C., whereas the surface of a soda-lime glass or of a silicon oxycarbide sublayer rather induces an anatase growth, in particular for deposition temperatures of the order of 400.degree. to 600.degree. C. All these optional thin layers can, in a known way, be deposited by vacuum techniques of the cathodic sputtering type or by other techniques of the thermal decomposition type, such as solid, liquid or gas phase pyrolyses. Each of the abovementioned layers can combine a number of functions but it is also possible to superimpose them. Another subject of the invention is "dirt-repellent" (organic and/or inorganic dirty marks) and/or "anti-condensation" glazing, whether it is monolithic or insulating multiple units of the double glazing or laminated type, which incorporates the coated substrates described above. The invention is thus targeted at the manufacture of glass, ceramic or vitroceramic products and very particularly at the manufacture of "self-cleaning" glazing. The latter can advantageously be building glazing, such as double glazing (it is then possible to arrange the coating "external side" and/or "internal side", that is to say on face 1 and/or on face 4). This proves to be very particularly advantageous for glazing which is not very accessible to cleaning and/or which needs to be cleaned very frequently, such as roofing glazing, airport glazing, and the like. It can also relate to vehicle windows where maintenance of visibility is an essential safety criterion. This coating can thus be deposited on car windscreens, side windows or rear windows, in particular on the face of the windows turned towards the inside of the passenger compartment. This coating can then prevent the formation of condensation and/or remove traces of dirty finger mark, nicotine or organic material type, the organic material being of the volatile plasticizing type released by the plastic lining the interior of the passenger compartment, in particular that of the dashboard (release sometimes known under the term "fogging"). Other vehicles such as planes or trains can also find it advantageous to use windows furnished with the coating of the invention. A number of other applications are possible, in particular for aquarium glass, shop windows, greenhouses, verandas, or glass used in interior furniture or street furniture but also mirrors, television screens, the spectacle field or any architectural material of the facing material, cladding material or roofing material type, such as tiles, and the like. The invention thus makes it possible to functionalize these known products by conferring on them anti-ultraviolet, dirt-repellent, bactericidal, anti-glare, anti-static or antimicrobial properties and the like. Another advantageous application of the coating according to the invention consists in combining it with an electrically controlled variable absorption glazing of the following types: electrochromic glazing, liquid crystal glazing, optionally with dichroic dye, glazing containing a system of suspended particles, viologen glazing and the like. As all these glazing types are generally composed of a plurality of transparent substrates, between which are arranged the "active" elements, it is then possible advantageously to arrange the coating on the external face of at least one of these substrates. In particular in the case of an electrochromic glazing, when the latter is in the coloured state, its absorption results in a degree of surface heating which, in fact, is capable of accelerating the photocatalytic decomposition of the carbonaceous substances which are deposited on the coating according to the invention. For further details on the structure of an electrochromic glazing, reference will advantageously be made to Patent Application EP-A-0,575,207, which describes an electrochromic laminated double glazing, it being possible for the coating according to the invention preferably to be positioned on face 1. Another subject of the invention is the various processes for obtaining the coating according to the invention. It is possible to use a deposition technique of the pyrolysis type which is advantageous because it in particular makes possible the continuous deposition of the coating directly on the float-glass strip when a glass substrate is used. The pyrolysis can be carried out in the solid phase, from powder(s) of precursor(s) of the organo-metallic type. The pyrolysis can be carried out in the liquid phase, from a solution comprising an organometallic titanium precursor of the titanium chelate and/or titanium alcoholate type. Such precursors are mixed with at least one other organometallic precursor. For further details on the nature of the titanium precursor or on the deposition conditions, reference will be made, for example, to Patents FR-2,310,977 and EP-0,465,309. The pyrolysis can also be carried out in the vapour phase, which technique is also denoted under the term of CVD (Chemical Vapour Deposition), from at least one titanium precursor of the halide type, such as TiCl.sub.4, or titanium alcoholate of the Ti tetraisopropylate type, Ti(OiPr).sub.4. The crystallization of the layer can additionally be controlled by the type of sublayer, as mentioned above. It is also possible to deposit the coating by other techniques, in particular by techniques in combination with the "sol-gel". Various deposition methods are possible, such as "dipping", also known as "dip coating", or a deposition using a cell known as "cell coating". It can also concern a method of deposition by "spray coating" or by laminar coating, the latter technique being described in detail in Patent Application WO-94/01598. All these deposition methods in general use a solution comprising at least one organometallic precursor, in particular titanium of the alcoholate type, which is thermally decomposed after coating the substrate with the solution on one of its faces or on both its faces. It can be advantageous, moreover, to deposit the coating, whatever the deposition technique envisaged, not in a single step but via at least two successive stages, which appears to promote the crystallization of titanium oxide throughout the thickness of the coating when a relatively thick coating is chosen. Likewise, it is advantageous to subject the coating with a photocatalytic property, after deposition, to a heat treatment of the annealing type. A heat treatment is essential for a technique of the sol-gel or laminar coating type in order to decompose the organometallic precursor(s) to oxide, once the substrate has been coated, and to improve the resistance to abrasion, which is not the case when a pyrolysis technique is used, where the precursor decomposes as soon as it comes into contact with the substrate. In the first case, as in the second, however, a post-deposition heat treatment, once the TiO.sub.2 has been formed, improves its degree of crystallization. The chosen treatment temperature can in addition make possible better control of the degree of crystallization and of the crystalline nature, anatase and/or rutile, of the oxide. However, in the case of a substrate made of soda-lime glass, multiple and prolonged annealings can promote attenuation of the photocatalytic activity because of an excessive migration of the alkali metals from the substrate towards the photoreactive layer. The use of a barrier layer between the substrate, if it is made of standard glass, and the coating, or the choice of a substrate made of glass with an appropriate composition, or alternatively the choice of a soda-lime glass with a surface from which alkali metals have been eliminated make it possible to remove this risk. Other advantageous details and characteristics of the invention emerge from the description below of non-limiting implementational examples, with the help of the following figures: FIG. 1: a cross-section of a glass substrate provided with the coating according to the invention, FIG. 2: a diagram of a sol-gel deposition technique, by so-called "dip coating" the coating, FIG. 3: a diagram of a so-called "cell coating" deposition technique, FIG. 4: a diagram of a so-called "spray coating" deposition technique, FIG. 5: a diagram of a deposition technique by laminar coating. As represented very diagrammatically in FIG. 1, all the following examples relate to the deposition of a so-called "dirt-repellent" coating 3, essentially based on titanium oxide, on a transparent substrate 1. The substrate 1 is made of clear soda-lime-silica glass with a thickness of 4 mm and a length and width of 50 cm. It is obvious that the invention is not limited to this specific type of glass. The glass can in addition not be flat but bent. Between the coating 3 and the substrate 1 is found a thin optional layer 2, either based on silicon oxycarbide, written as SiOC, for the purpose of constituting a barrier to the diffusion of the alkali metals and/or a layer which attenuates light reflection, or based on tin oxide doped with fluorine SnO.sub.2 :F, for the purpose of constituting an anti-static and/or low-emissive layer, even with a not very pronounced low-emissive effect, and/or a layer which attenuates the colour, in particular in reflection. EXAMPLES 1 TO 3 Examples 1 to 3 relate to a coating 3 deposited using a liquid phase pyrolysis technique. The operation can be carried out continuously, by using a suitable distribution nozzle arranged transversely and above the float-glass strip at the outlet of the float-bath chamber proper. In this instance, the operation is carried out non-continuously, by using a moveable nozzle arranged opposite the substrate 1 already cut to the dimensions shown, which substrate is first heated in an oven to a temperature of 400 to 650.degree. C. before progressing a constant speed past the nozzle spraying at an appropriate solution. Example 1 In this example, there is no optional layer 2. The coating 3 is deposited using a solution comprising two organometallic titanium precursors, titanium diisopropoxide diacetylacetonate and titanium tetraoctyleneglycolate, dissolved in a mixture of two solvents, the latter being ethyl acetate and isopropanol. It should be noted that it is also entirely possible to use other precursors of the same type, in particular other titanium chelates of the titanium acetylacetonate, titanium (methyl acetoacetato), titanium (ethyl acetoacetato) or alternatively titanium triethanolaminato or titanium diethanolaminato type. As soon as the substrate 1 has reached the desired temperature in the oven, i.e. in particular approximately 500.degree. C., the substrate progresses past the nozzle which sprays at room temperature, using compressed air, the mixture shown. A TiO.sub.2 layer with a thickness of approximately 90 nm is then obtained, it being possible for the thickness to be controlled by the rate of progression of the substrate 1 past the nozzle and/or the temperature of the said substrate. The layer is partially crystalline in the anatase form. This layer exhibits excellent mechanical behaviour. Its resistance to abrasion tests is comparable with that obtained for the surface of the bare glass. It can be bent and dip coated. It does not exhibit bloom: the scattered light transmission of the coated substrate is less than 0.6% (measured according to the D.sub.65 illuminant at 560 nm) Example 2 Example 1 is repeated but inserting, between the substrate 1 and coating 3, an SnO.sub.2 :F layer 2 with a thickness of 73 nm. This layer is obtained by powder pyrolysis from dibutyltin difluoride DBTF. It can also be obtained, in a known way, by pyrolysis in the liquid or vapour phase, as is for example described in Patent Application EP-A-0,648,196. In the vapour phase, it is possible in particular to use a mixture of monobutyltin trichloride and of a fluorinated precursor optionally in combination with a "mild" oxidant of the H.sub.2 O type. The index of the layer obtained is approximately 1.9. Its surface resistance is approximately 50 ohms. In the preceding Example 1, the coated substrate 1, mounted as a double glazing so that the coating is on face 1 (with another substrate 1' which is non-coated but of the same nature and dimensions as the substrate 1 via a 12 mm layer of air), exerts a colour saturation value in reflection of 26% and a colour saturation value in transmission of 6.8%. In this Example 2, the colour saturation in reflection (in the goldens) is only 3.6% and it is 1.1% in transmission. Thus, the SnO.sub.2 :F sublayer makes it possible to confer, on the substrate, anti-static properties due to its electrical conductivity and it also has a favourable effect on the colorimetry of the substrate, by making its coloration markedly more "neutral", both in transmission and in reflection, which coloration is caused by the presence of the titanium oxide coating 3 exhibiting a relatively high refractive index. It is possible to polarize it by providing it with a suitable electrical supply, in order to limit the deposition of dust with a relatively large size, of the order of a millimetre. In addition, this sublayer decreases the diffusion of alkali metals into the photocatalytic TiO.sub.2 layer. The photocatalytic activity is thus improved. Example 3 Example 2 is repeated but this time inserting, between substrate 1 and coating 3, a layer 2 based on silicon oxycarbide with an index of approximately 1.75 and a thickness of approximately 50 nm, which layer can be obtained by CVD from a mixture of SiH.sub.4 and ethylene diluted in nitrogen, as described in Patent Application EP-A-0,518,755. This layer is particularly effective in preventing the tendency of alkali metals (Na.sup.+, K.sup.+) and of alkaline-earth metals (Ca.sup.++) originating from the substrate 1 to diffuse towards the coating 3 and thus the photocatalytic activity is markedly improved. As it has, like SnO.sub.2 :F, a refractive index intermediate between that of the substrate (1.52) and of the coating 3 (approximately 2.30 to 2.35), it also makes it possible to reduce the intensity of the coloration of the substrate, both in reflection and in transmission, and overall to decrease the light reflection value R.sup.L of the said substrate. The following Examples 4 to 7 relate to depositions by CVD. EXAMPLES 4 TO 7 Example 4 This example relates to the deposition by CVD of the coating 3 directly on the substrate 1 using a standard nozzle, such as that represented in the above-mentioned Patent Application EP-A-0,518,755. Use is made, as precursors, either of an organometallic compound or of a metal halide. In this instance, titanium tetraisopropylate is chosen as organometallic compound, this compound being advantageous because of its high volatility and its large working temperature range, from 300 to 650.degree. C. In this example, deposition is carried out at approximately 425.degree. C. and the TiO.sub.2 thickness is 15 nm. Tetraethoxytitanium Ti(O--Et).sub.4 may also be suitable and, as halide, mention may be made of TiCl.sub.4. Example 5 It is carried out similarly to Example 4, except that, in this instance, the 15 nm TiO.sub.2 layer is not deposited directly on the glass but on a 50 nm SiOC sublayer deposited as in Example 3. Example 6 It s carried out as in Example 4, except that, in this instance, the thickness of the TiO.sub.2 layer is 65 nm. Example 7 It is carried out as in Example 5, except that, in this instance, the thickness of the TiO.sub.2 layer is 60 nm. From these Examples 4 to 7, it is found that the substrates thus coated exhibit good mechanical behaviour with respect to the abrasion tests. In particular, no delamination of the TiO.sub.2 layer is observed. Example 8 This example uses a technique in combination with the sol-gel using a deposition method by "dipping", also known as "dip coating", the principle of which emerges from FIG. 2: it consists in immersing the substrate 1 in the liquid solution 4 containing the appropriate precursor(s) of the coating 3 and in then withdrawing the substrate 1 therefrom at a controlled rate using a motor means 5, the choice of the rate of withdrawal making it possible to adjust the thickness of solution remaining at the surface of the two faces of the substrate and, in fact, the thickness of the coatings deposited, after heat treatment of the latter in order both to evaporate the solvent and to decompose the precursor or precursors to oxide. Use is made, for depositing the coating 3, of a solution 4 comprising either titanium tetrabutoxide Ti(O--Bu).sub.4, stabilized with diethanolamine DEA in the molar proportion 1:1, in an ethanol-type solvent containing 0.2 mol of tetrabutoxide per litre of ethanol, or the mixture of precursors and of solvents described in Example 1. (Another precursor, such as titanium (diethanolaminato)dibutoxide, can also be used). The substrates 1 can contain SiOC sublayers. After withdrawal from each of the solutions 4, the substrates 1 are heated for 1 hour at 100.degree. C. and then for approximately 3 hours at 550.degree. C. with the temperature raised gradually. A coating 3 is obtained on each of the faces, which coating is in both cases made of highly crystalline TiO.sub.2 in the anatase form. Example 9 This example uses the technique known as "cell coating", the principle of which is recalled in FIG. 3. It relates to forming a narrow cavity, delimited by two substantially parallel faces 6, 7 and two seals 8, 9, at least one of these faces 6, 7 consisting of the face of the substrate 1 to be treated. The cavity is then filled with the solution 4 of precursor(s) of the coating and the solution 4 is withdrawn in a controlled way, so as to form a wetting meniscus, for example using a peristaltic pump 10, leaving a film of the solution 4 on the face of the substrate 1 as this solution is withdrawn. The cavity 5 is then maintained for at least the time necessary for drying. The film is cured by heat treatment. The advantage of this technique, in comparison with "dip coating", is in particular that it is possible to treat only a single one of the two faces of the substrate 1 and not both systematically, unless a masking system is resorted to. The substrates 1 comprise thin layers 2 based on silicon oxycarbide SiOC. Example 6 uses respectively the solutions 4 described in Example 8. The same heat treatments are then carried out in order to obtain the TiO.sub.2 coating 3. The coating 3 exhibits good mechanical durability. Under an SEM (scanning electron microscope), a field effect appears in the form of "grains" of monocrystals with a diameter of approximately 30 nm. The roughness of this coating induces wetting properties which are enhanced with respect to a non-rough coating. These same solutions 4 can also be used to deposit coatings by "spray coating", as represented in FIG. 4, where the solution 4 is sprayed in the form of a cloud against the substrate 1 statically, or by laminar coating, as represented in FIG. 5. In the latter case, the substrate 1, held by vacuum suction against a support 11 made of stainless steel and Teflon, is passed over a tank 12 containing the solution, in which solution is partially immersed a slotted cylinder 14, and the combined tank 12 and cylinder 14 are then moved over the whole length of the substrate 1, the mask 13 preventing excessive evaporation of the solvent from the solution 4. For further details regarding this latter technique, reference will advantageously be made to the abovementioned Patent Application WO-94/01598. Tests were carried out on the substrates obtained according to the above examples in order to characterize the coatings deposited and to evaluate their "anti-condensation" and "dirt-repellent" behaviour. Test 1: This is the test of the condensation aspects. It consists in observing the consequences of the photocatalysis and of the structure of the coating (level of hydroxyl groups, porosity, roughness) on the wetting. If the surface is photoreactive, the carbonaceous microcontaminants which are deposited on the coating are continually destroyed and the surface is hydrophilic and thus anti-condensation. It is also possible to carry out a quantitative evaluation by suddenly reheating the initially coated substrate, stored in the cold or simply by blowing over the substrate, by measuring if condensation appears and, in the affirmative, at what time, and by then measuring the time necessary for the disappearance of the said condensation. Test 2: It relates to the evaluation of the hydrophilicity and the oleophilicity at the surface of the coating 3, in comparison with those of the surface of a bare glass, by measurement of contact angles of a drop of water and of a drop of DOP (dioctyl phthalate) at their surfaces, after having left the substrates for one week in the surrounding atmosphere under natural light, in the dark and then having subjected them to UVA radiation for 20 minutes. Test 3: It consists in depositing, on the substrate to be evaluated, a layer of an organosilane and in irradiating it with UVA radiation so as to degrade it by photocatalysis. As the organosilane modifies the wetting properties, measurements of contact angle of the substrate with water during the irradiation indicate the state of degradation of the grafted layer. The rate of disappearance of this layer is related to the photocatalytic activity of the substrate. The grafted organosilane is a trichlorosilane: octadecyltrichlorosilane (OTS). The grafting is carried out by dipping. The test device is composed of a turntable rotating around from 1 to 6 low pressure UVA lamps. The test specimens to be evaluated are placed in the turntable, the face to be evaluated on the side of the UVA radiation. Depending on their position and the number of lamps switched on, each test specimen receives a UVA irradiation varying from 0.5 W/m.sup.2 to 50 W/m.sup.2. For Examples 1, 2, 3, 8 and 9, the irradiation power is chosen as 1.8 W/m.sup.2 and, for Examples 4 to 7, as 0.6 W/m.sup.2. The time between each measurement of the contact angle varies between 20 min and 3 h, depending on the photocatalytic activity of the test specimen under consideration. The measurements are carried out using a goniometer. Before irradiation, the glasses exhibit an angle of approximately 100.degree.. It is considered that the layer is destroyed after irradiation when the angle is less than 20.degree.. Each test specimen tested is characterized by the mean rate of disappearance of the layer, given in nanometres per hour, that is to say the thickness of the organosilane layer deposited divided by the irradiation time which makes it possible to reach a final stationary value of less than 20.degree. (time for disappearance of the organosilane layer) All the preceding examples pass Test 1, that is to say that, when the substrates coated with the coating are blown on, they remain perfectly transparent, whereas a highly visible layer of condensation is deposited on non-coated substrates. The examples were subjected to Test 2: the coated substrates, after exposure to UVA radiation, exhibit a contact angle with water and with DOP of not more than 50. In contrast, a bare glass under the same conditions exhibits a contact angle with water of 40.degree. and a contact angle with DOP of 20.degree.. The results of the substrates coated according to the above examples in Test 3 are combined in the table below. Test 3, of wetting, at 1.8 W/m.sup.2 UVA Substrate (in nm/h) Example 1 (TiO.sub.2 on bare glass) 0.03 Example 2 (TiO.sub.2 on SnO.sub.2 :F) 0.1 Example 3 (TiO.sub.2 on SiOC) 0.2 Example 8 (TiO.sub.2 on 50 nm SiOC) 5 Example 9 (TiO.sub.2 on 50 nm SiOC) 5 Bare glass 0 Test 3, of wetting, at 0.6 W/m.sup.2 UVA Substrate (CVD) (in nm/h) Example 4 (TiO.sub.2 on bare glass) <0.05 nm/h Example 5 (TiO.sub.2 on SiOC) 4 Example 6 (TiO.sub.2 on bare glass) Example 7 (TiO.sub.2 on SiOC) 9 19.5 From the table, it can be seen that the presence of sublayers, in particular of SiOC, promotes the photocatalytic activity of the coating containing the TiO.sub.2, by its barrier effect to alkali metals and alkaline-earth metals which can migrate from the glass (comparison of Examples 4 and 5 or 6 and 7). It is also observed that the thickness of the coating containing the TiO.sub.2 also plays a role (comparison of Examples 1 and 3) for a TiO.sub.2 coating with a thickness greater than the mean size of the monocrystals or "crystallites", a better photocatalytic effect is obtained. Indeed, it could be observed that the TiO.sub.2 coatings obtained by CVD exhibit the most advanced crystallization, with crystallite sizes of the order of 20 to 30 nm. It can be seen that the photocatalytic activity of Example 6 (65 nm of TiO.sub.2) is markedly greater than that of Example 4 (15 nm of TiO.sub.2 only). It is therefore advantageous to provide a TiO.sub.2 coating thickness at least two times greater than the mean diameter of the crystallites which it contains. Alternatively, as in the case of Example 5, it is possible to retain a TiO.sub.2 coating with a thin thickness but then to choose to use a sublayer of an appropriate nature and with an appropriate thickness for promoting as far as possible the crystalline growth of TiO.sub.2 from the "first" layer of crystallites. It could be observed that the crystallization of the TiO.sub.2 was somewhat less advanced for the coatings deposited by a technique other than CVD. Here again, however, everything is still a matter of compromise: a less advanced crystallization and an a priori lower photocatalytic activity can be "compensated for" by the use of a deposition process which is less expensive or less complex, for example. Moreover, the use of an appropriate sublayer or the doping of the TiO.sub.2 can make it possible to improve the photocatalytic behaviour, if necessary. It is also confirmed, from the comparison of Examples 2 and 3, that the nature of the sublayer influences the crystallization form and, in fact, the photocatalytic activity of the coating.