Molecular Pathology Core
advertisement

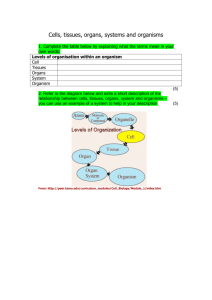
Molecular Pathology Core Submission Instructions A. How do I fill out the Submission form? Download the submission form to your computer. Complete the form and email to molecular@ufl.edu. First time submitters also need to complete and email the Account Registration form. B. How do I submit the samples? Please read the excellent information on UAB website . Trim tissues to fit in cassettes. We have regular, large, and biopsy cassettes as well as mesh bags and blue sponges that are free to our users in D11-41. You can also get them labeled by submitting a cassette request form. The tissue surface that is place down in the cassette will be the surface that is sectioned unless you write special requests. Use only a #2 pencil for labeling cassettes. Solvents used in processing can remove the ink labels made from most Sharpie markers. To prevent small tissues from being lost during processing, place them in biopsy cassettes or between filter paper. The processor uses vacuum to facilitate tissue infiltration and this can coax small tissues out of the cassette. Refrain from overcrowding tissues in the cassette. Tissue that is compressed in the cassette will not adequately infiltrate or section well later on. Specimens should be cut thin enough so that the fixative penetrates the tissue within a reasonably short time. Allow ~1mm/hour for the fixative to penetrate your tissues and a volume of 10-20 times fixative volume to tissue. The thickness and size of a nickel or 5mm is a good guide to use when trimming samples. If batches of tissues are being submitted at the same time that vary significantly in size (whole organs vs biopsy samples), sort by size or tissue consistency (ie. all solid organs), and submit in separate cassettes. Avoid over-fixation particularly if immunolocalization is needed. Keep cassettes submerged until processed. End fixation in your lab then transfer to PBS or 70% ethanol. Transport samples in a leak-proof container. Make sure it is labeled with your name, PI and solution. C. Brief guide for specific organs and fixatives (see links on Research Histology webpage): Bone: Femur and sternum are good bones to harvest. Open ends of femur to improve fixative penetration. Decalcification: After fixation, bone and other calcified tissues must be treated to remove the calcium content. We use RDO Rapid Decalcifier from Apex Engineering (Cat.#RDO01). Liver: Remove entire organ from body cavity, weigh if needed, then cut a transverse section through the largest lobe and then through the two lobes including the gallbladder. Avoid over-fixation and loss of glycogen (Swiss cheese morphology). Intestinal tract: Remove from body cavity, flush out with PBS or open entire length, place about 1 to 1.5 cm sections each of small intestine and large intestine on filter paper to maintain linearity. You can also roll up the entire tract as a "Swiss roll". Stomach- open along greater curvature, rinse off ingesta, make longitudinal cuts that include the distal portion (antrum) into duodenum. Lay between 2 papers to fix flat then have embedded on edge. Lung: Remove lungs with trachea intact from body cavity, re-inflate with fixative (insert needle on syringe into trachea, pre-place a suture, re- inflate lungs gently. Remove needle and snug trachea suture tight.). Immerse inflated lungs in fixative for 30 min-overnight, then submit all or take coronal sections through the largest cross section from each side for cassette. Heart- Remove with lungs, can fix as a pluck or remove from lungs and take longitudinal section to include valves, atria and ventricular walls. Skin: Collect from least furry area. The presence of abnormalities may dictate an alternative site. Obtain ~ 5 mm long and 1 mm wide rectangle of skin, place between filter papers to fix flat, then put in cassette. Brain and testis: Best to immersion fix overnight before trimming and placing in cassettes. Bouin's fixative is preferred for testis. Lesions: Always take anything that seems out of the ordinary. Include adjacent normal tissue for comparison. D. Fixatives (Fred Hutchinson Cancer Research Center) Fresh tissues should be rinsed with PBS to remove any blood or foreign material then immediately transferred to cassettes and immersed in fixative to avoid autolysis. Intestine, pancreas, liver, and brain are particularly prone to autolysis. Other organs can be kept a short time in cold PBS. Some organs are better trimmed to cassettes after fixation (brain, lung, heart, testis). If RNA is to be purified, the tissues should be harvested quickly, trimmed, placed into microfuge tubes, and snap frozen in liquid nitrogen. RNAlater (Ambion) can be put into tubes before harvesting and tissue sections minced in the tube before freezing to facilitate homogenization and penetration. Sample sectioning: Samples are routinely sectioned at 4-6 microns when placed on glass slides. Thicker sections may be desired if mapping structures across many sections. When extracting paraffinized material for DNA or RNA extraction, there are two methods that are utilized. First, unstained slides may be prepared, deparaffinized in xylene, and scraped into containers. Second, unmounted paraffin “scrolls” may be cut and placed into containers for further processing. If RNA is the product of interest, it is important to notify the laboratory so that special procedures can be utilized that prevent cross-contamination. Formalin: Fixation time 12-48 hours. Formalin solution, 10% neutral buffered (Fisher, 23-245-684). This contains 4% w/v formaldehyde with phosphate buffers. Formalin stabilizes proteins and prevents decomposition. It is very good for preserving morphology and generally good for IHC. Mouse tissues can be fixed several hours to overnight, however, you will need to determine what is optimal for your particular needs. 4% paraformaldehyde in PBS: Fixation time depends on technique to follow. Paraformaldeyde is the anhydrous form of formaldehyde. It needs to be made fresh in PBS or stored in frozen aliquots. It is less harsh than 10% formalin and is preferred for many antigens in immunostaining, particularly direct GFP detection and CNS . PLP: Fixation time 24-48 hours at 4C. 2% paraformaldehyde, excellent for IHC. Tissues are softer as fixation minimal and may be more difficult to section. Bouin's: Fixation time 6 hours. This fixative can be purchased and is sometimes preferred for immunostaining with certain antibodies. After fixation, rinse tissues in 70% ethanol to remove yellow color before processing with other tissues.