Arterial pressure at children depending on age
advertisement

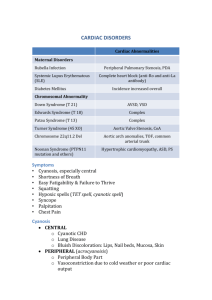
Cardiovascular system includes heart, coronary vessels, the main vessels (an aorta, a pulmonary trunk), capillaries. The major functions of organs of a circulation are maintenance of a constancy of internal medium of an organism: delivery of oxygen and nutrients to all organs and tissues, excision and allocation of Carbonei dioxydum and other products of an exchange from an organism. Cardiovascular system starts to be pawned on 2 - 3 week of the pre-natal period. To the extremity of 1 month begins, and within 2 months formation of both half of heart proceeds. In the end of 4 weeks interatrial and interventricular septums start to be formed. On 7 week near to a primary interatrial septum it is formed secondary, a primary oval aperture in such a manner that the blood current to become possible only in one direction - from the right auricle in left. The valval apparatus of heart arises after formation of septums. From the extremity of 5 weeks the primary system of a circulation of an embryos starts to function. The fetus circulation has chorial type and is independent after the relation to a circulation of mother. Blood on an umbilical vein gets to a foetus where it is referred to a liver. Before occurrence in it through wide and short venous the duct gives an essential part of blood to the bottom vena cava, and then is bridged to a portal vein. Having passed through a liver, the admixed blood through the bottom vena cava gets to the right auricle, and then through an oval window in the left auricle, a left ventricle and on an aorta in the big circle of a circulation. Other part of the admixed blood from the bottom vena cava gets through the right auricle to a right ventricle and on a pulmonary trunk is referred to lungs. Through the arterial duct bridging a pulmonary artery with an aorta, the blood most part is dumped in the big circle of a circulation. The blood of the descending aorta which have given oxygen, on umbilical arteries comes back in a capillary network of chorial villuses of a placenta. At a birth there is a circulation reorganisation: The placental circulation stops; The basic are closed fetal vascular communications; In full the vascular bed of a small circle of a circulation joins; Because of augmentation of requirement for oxygen emission and system vascular pressure accrue warm. The importance of the history and physical examination cannot be overemphasized in the evaluation of infants and children with suspected cardiovascular disorders. Patients may require further laboratory evaluation and eventual treatment, or the family may be reassured that no significant problem exists. Although the ready availability of echocardiography may entice the clinician to skip these preliminary steps, an initial evaluation by a skilled cardiologist is preferred for several reasons: a cardiac examination allows the cardiologist to guide the echocardiographic evaluation toward confirming or eliminating specific diagnoses, thereby increasing its accuracy; because most childhood murmurs are innocent, evaluation by a pediatric cardiologist can eliminate unnecessary and expensive laboratory tests; and the cardiologist's knowledge and experience are important in reassuring the patient's family and preventing unnecessary restrictions on healthy physical activity. History. A comprehensive cardiac history starts with details of the perinatal period, including the presence of cyanosis, respiratory distress, or prematurity. Maternal complications such as gestational diabetes, medications, systemic lupus erythematosus, or substance abuse can be associated with cardiac problems. If cardiac symptoms began during infancy, the timing of the initial symptoms should be noted to provide important clues about the specific cardiac condition. Many of the symptoms of congestive heart failure in infants and children are age specific. In infants, feeding difficulties are common. Inquiry should be made about the frequency of feeding and either the volume of each feeding or the time spent on each breast. An infant with heart failure often takes less volume per feeding and become dyspneic or diaphoretic while sucking. After falling asleep exhausted, the baby, inadequately fed, will awaken for the next feeding after a brief time. This cycle continues around the clock and must be carefully differentiated from colic or other feeding disorders. Additional symptoms and signs include those of respiratory distress: rapid breathing, nasal flaring, cyanosis, and chest retractions. In older children, heart failure may be manifested as exercise intolerance, difficulty keeping up with peers during sports or need for a nap after coming home from school, and poor growth. Eliciting a history of fatigue in an older child requires questions about age-specific activities, including stair climbing, walking, bicycle riding, physical education class, and competitive sports; information should be obtained regarding more severe manifestations such as orthopnea and nocturnal dyspnea. Cyanosis at rest is often overlooked by parents; it may be mistaken for a normal individual variation in coloration. Cyanosis during crying or exercise, however, is more often noted as abnormal by observant parents. Many infants and toddlers turn “blue around the lips” when crying vigorously or during breath-holding spells; this condition must be carefully differentiated from cyanotic heart disease by inquiring about inciting factors, the length of episodes, and whether the tongue and mucous membranes also appear cyanotic. Newborns have cyanotic extremities (acrocyanosis) when undressed and cold, and this response to cold must be carefully differentiated from true cyanosis. Chest pain is an unusual manifestation of cardiac disease in pediatric patients, although it is a frequent cause for referral to a pediatric cardiologist, especially in adolescents. Nonetheless, a careful history, physical examination, and if indicated, laboratory or imaging tests will assist in identifying the cause of chest pain. Cardiac disease may be a manifestation of a known congenital malformation syndrome with typical physical findings or a manifestation of a generalized disorder affecting the heart and other organ systems. Extracardiac malformations may be noted in 20–45% of infants with congenital heart disease. Between 5 and 10% of patients have a known chromosomal abnormality; this percentage will increase as our knowledge of specific gene defects linked to congenital heart disease increases. A careful family history may also reveal early coronary artery disease or stroke (familial hypercholesterolemia or thrombophilia), generalized muscle disease (muscular dystrophy, dermatomyositis, familial or metabolic cardiomyopathy), or relatives with congenital heart disease. General Physical Examination. After general assessment of the patient, specific attention is directed toward the presence of cyanosis, abnormalities in growth, and any evidence of respiratory distress. Evaluation of a murmur must always be performed in the context of other physical findings. Frequently, associated findings, such as the quality of the pulses or the presence of a ventricular heave, provide important clues to a specific cardiac diagnosis. Accurate measurement of height and weight and plotting on a standard growth chart are important because both cardiac failure and chronic cyanosis result in failure to thrive. Growth failure is usually manifested predominantly by poor weight gain; if length or head circumference is also affected, additional congenital malformations or metabolic disorders may be present. Mild cyanosis may be too subtle for early detection, and clubbing of the fingers and toes is not usually manifested until late in the 1st yr of life, even in the presence of severe arterial oxygen desaturation. Cyanosis is best observed over the nail beds, lips, tongue, and mucous membranes. Differential cyanosis, manifested as blue lower extremities and pink upper extremities (usually the right arm), is seen with right-to-left shunting across a ductus arteriosus in the presence of coarctation or an interrupted aortic arch. Circumoral cyanosis or blueness around the forehead may be the result of prominent venous plexuses in these areas rather than decreased arterial oxygen saturation. The extremities of infants often turn blue when the infant is unwrapped and cold (acrocyanosis), and this condition can be distinguished from central cyanosis by examination of the tongue and mucous membranes. Heart failure in infants and children results in some degree of hepatomegaly and occasionally splenomegaly. The sites of peripheral edema are age dependent. In infants, edema is usually seen around the eyes and over the flanks, especially after first waking in the morning. Older children and teenagers manifest both periorbital edema and pedal edema. A frequent initial complaint in these older patients is that their clothes no longer fit. The heart rate of newborn infants is rapid and subject to wide fluctuations. The average rate ranges from 120 to 140 beats/min and may increase to 170+ beats/min during crying and activity or drop to 70–90 beats/min during sleep. As the child grows older, the average pulse rate decreases and may be as low as 40 beats/min in athletic adolescents. Persistent tachycardia (>200 beats/min in neonates, 150 beats/min in infants, or 120 beats/min in older children), bradycardia, or an irregular heartbeat other than sinus arrhythmia requires investigation to exclude pathologic arrhythmias. Careful evaluation of the character of the pulses is an important early step in the physical diagnosis of congenital heart disease. A wide pulse pressure with bounding pulses may suggest an aortic runoff lesion such as patent ductus arteriosus, aortic insufficiency, an arterial-venous communication, or increased cardiac output secondary to anemia, anxiety, or conditions associated with increased catecholamine or thyroid hormone secretion. The presence of diminished pulses in all extremities is associated with pericardial tamponade, left ventricular outflow obstruction, or cardiomyopathy. The radial and femoral pulses should always be palpated simultaneously. Normally, the femoral pulse should be appreciated immediately before the radial pulse. In older children with coarctation of the aorta, blood flow to the descending aorta may channel through collateral vessels and result in the femoral pulse being delayed until after the radial pulse (radial-femoral delay). Blood pressure should be measured in the arms as well as in the legs, the latter on at least one occasion to be certain that coarctation of the aorta is not overlooked. Palpation of the femoral or dorsalis pedis pulse, or both, is not reliable alone to exclude coarctation. In older children, a mercury sphygmomanometer with a cuff that covers approximately two thirds of the upper part of the arm or leg may be used for blood pressure measurement. A cuff that is too small results in falsely high readings, whereas a cuff that is too large records slightly decreased pressure. Pediatric clinical facilities should be equipped with 3, 5, 7, 12, and 18cm cuffs to accommodate the large spectrum of pediatric patient sizes. The 1st Korotkoff sounds indicate systolic pressure. As cuff pressure is slowly decreased, the sounds usually become muffled before they disappear. Diastolic pressure may be recorded when the sounds become muffled (preferred) or when they disappear altogether; the former is usually slightly higher and the latter slightly lower than true diastolic pressure. For lower extremity blood pressure determination, the stethoscope is placed over the popliteal artery. Ordinarily, the pressure recorded in the legs with the cuff technique is about 10mmHg higher than that in the arms. In infants, blood pressure can be determined by auscultation, palpation, or an oscillometric (Dinamap) device, which if used properly, provides accurate measurements in infants as well as older children. Blood pressure varies with the age of the child and is closely related to height and weight. Significant increases occur during adolescence, and many temporary variations take place before the more stable levels of adult life are attained. Exercise, excitement, coughing, crying, and struggling may raise the systolic pressure of infants and children as much as 40–50mmHg greater than their usual levels. Variability in blood pressure in children of approximately the same age and body build should be expected, and serial measurements should always be obtained when evaluating a patient with hypertension. Though of little use in infants, in cooperative older children, inspection of the jugular venous pulse wave provides information about central venous and right atrial pressure. The neck veins should be inspected with the patient sitting at a 90-degree angle. The external jugular vein should not be visible above the clavicles unless central venous pressure is elevated. Increased venous pressure transmitted to the internal jugular vein may appear as venous pulsations without visible distention; such pulsation is not seen in normal children reclining at an angle of 45 degrees. Because the great veins are in direct communication with the right atrium, changes in pressure and the volume of this chamber are also transmitted to the veins. The one exception occurs in superior vena cava obstruction, in which venous pulsatility is lost. Cardiac Examination. The heart should be examined in a systematic manner starting with inspection and palpation. A precordial bulge to the left of the sternum with increased precordial activity suggests cardiac enlargement; such bulges can often best be appreciated by having the child lay supine with the examiner looking up from the child's feet. A substernal thrust indicates the presence of right ventricular enlargement, whereas an apical heave is noted with left ventricular hypertrophy. A hyperdynamic precordium suggests a volume load such as that found with a large left-to-right shunt, although it may be normal in a thin patient. A silent precordium with a barely detectable apical impulse suggests pericardial effusion or severe cardiomyopathy; it may be normal in an obese patient. The relationship of the apical impulse to the midclavicular line is also helpful in the estimation of cardiac size: the apical impulse moves laterally and inferiorly with enlargement of the left ventricle. Right-sided apical impulses signify dextrocardia, tension pneumothorax, or left-sided thoracic space-occupying lesions (e.g., diaphragmatic hernia). Thrills are the palpable equivalent of murmurs and correlate with the area of maximal auscultatory intensity of the murmur. It is important to palpate the suprasternal notch and neck for aortic bruits, which may indicate the presence of aortic stenosis or, when faint, pulmonary stenosis. Right lower sternal border and apical systolic thrills are characteristic of ventricular septal defect and mitral insufficiency, respectively. Diastolic thrills are occasionally palpable in the presence of atrioventricular valve stenosis. The timing and localization of thrills should be carefully noted. Auscultation is an art that improves with practice. The diaphragm of the stethoscope is placed firmly on the chest for high-pitched sounds; a lightly placed bell is optimal for low-pitched sounds. The physician should initially concentrate on the characteristics of the individual heart sounds and their variation with respirations and later concentrate on murmurs. The patient should be supine, lying quietly, and breathing normally. The 1st heart sound is best heard at the apex, whereas the 2nd heart sound should be evaluated at the upper left and right sternal borders. The 1st heart sound is caused by closure of the atrioventricular valves (mitral and tricuspid); the 2nd sound is caused by closure of the semilunar valves. During inspiration, the decrease in intrathoracic pressure results in increased filling of the right side of the heart, which leads to an increased right ventricular ejection time and thus delayed closure of the pulmonary valve; consequently, splitting of the 2nd heart sound increases during inspiration and decreases during expiration. Often, the 2nd heart sound appears to be single during expiration. The presence of a normally split 2nd sound is strong evidence against the diagnosis of atrial septal defect, defects associated with pulmonary arterial hypertension, severe pulmonary valve stenosis, aortic and pulmonary atresia, and truncus arteriosus. Wide splitting is noted in atrial septal defect, pulmonary stenosis, Ebstein anomaly, total anomalous pulmonary venous return, and right bundle branch block. An accentuated pulmonic component of the 2nd sound with narrow splitting is a sign of pulmonary hypertension. A single 2nd sound occurs in pulmonary or aortic atresia or severe stenosis, truncus arteriosus, and often in transposition of the great arteries. A 3rd heart sound is best heard with the bell at the apex in mid-diastole. A 4th sound occurring in conjunction with atrial contraction may be heard just before the 1st heart sound in late diastole. The 3rd sound may be normal in an adolescent with a relatively slow heart rate, but in a patient with the clinical signs of heart failure and tachycardia, it may be heard as a gallop rhythm and may merge with a 4th heart sound, a finding known as a summation gallop. A gallop rhythm is attributed to poor compliance of the ventricle, and exaggeration of the normal 3rd sound is associated with ventricular filling. Ejection clicks, which are heard in early systole, may be related to dilatation of the aorta or pulmonary artery or to a mildly to moderately stenotic semilunar valve. They are heard so close to the 1st heart sound that they may be mistaken for a split 1st sound. Aortic ejection clicks are best heard at the left middle to right upper sternal border and are constant in intensity. They occur in conditions in which the aortic valve is stenotic or the aorta is dilated (tetralogy of Fallot, truncus arteriosus). Pulmonary ejection clicks, which are associated with mild to moderate pulmonary stenosis, are best heard at the left middle to upper sternal border and vary with respirations, often disappearing with inspiration. Split 1st heart sounds are usually heard best at the lower left sternal border. A midsystolic click heard at the apex, often preceding a late systolic murmur, suggests mitral valve prolapse. Murmurs should be described according to their intensity, pitch, timing (systolic or diastolic), variation in intensity, time to peak intensity, area of maximal intensity, and radiation to other areas. Auscultation for murmurs should be carried out across the upper precordium, down the left or right sternal border, and out to the apex and left axilla. Auscultation should also always be performed in the right axilla and over the back. Systolic murmurs are classified as ejection, pansystolic, or late systolic according to the timing of the murmur in relation to the 1st and 2nd heart sounds. The intensity of systolic murmurs is graded from I to VI: I, barely audible; II, medium intensity; III, loud but no thrill; IV, loud with a thrill; V, very loud but still requiring positioning of the stethoscope at least partly on the chest; and VI, so loud that the murmur can be heard with the stethoscope off the chest. Systolic ejection murmurs start a short time after a well-heard 1st heart sound, increase in intensity, peak, and then decrease in intensity; they usually end before the 2nd sound. However, in patients with severe aortic or pulmonary stenosis, the murmur may extend beyond the 1st component of the 2nd sound, thus obscuring it. Pansystolic or holosystolic murmurs begin almost simultaneously with the 1st heart sound and continue throughout systole, on occasion becoming gradually decrescendo. It is helpful to remember that after closure of the atrioventricular valves (the 1st heart sound), a brief period occurs during which ventricular pressure increases but the semilunar valves remain closed. Thus, pansystolic murmurs (heard during both isovolumic contraction and the ejection phases of systole) cannot be caused by flow across the semilunar valves because these valves are closed during isovolumic contraction. Pansystolic murmurs are therefore related to blood exiting the contracting ventricle via either an abnormal opening (a ventricular septal defect) or atrioventricular (mitral or tricuspid) valve insufficiency. Systolic ejection murmurs usually imply increased flow or stenosis across one of the ventricular outflow tracts (aortic or pulmonic). In infants with rapid heart rates, it is often difficult to distinguish between ejection and pansystolic murmurs. If a clear and distinct 1st heart sound can be appreciated, the murmur is most likely ejection in nature. A continuous murmur is a systolic murmur that continues or “spills” into diastole and indicates continuous flow, such as in the presence of a patent ductus arteriosus or other aortopulmonary communication. This murmur should be differentiated from a to-and-fro murmur, which indicates that the systolic component of the murmur ends at or before the 2nd sound and the diastolic murmur begins after semilunar valve closure (e.g., aortic or pulmonary stenosis combined with insufficiency). A late systolic murmur begins well beyond the 1st heart sound and continues until the end of systole. Such murmurs may be heard after a midsystolic click in patients with mitral valve prolapse and insufficiency. Several types of diastolic murmurs (graded I–IV) can be identified. A decrescendo diastolic murmur is a blowing murmur along the left sternal border that begins with S2 and diminishes toward mid-diastole. When high-pitched, this murmur is associated with aortic valve insufficiency or pulmonary insufficiency related to pulmonary hypertension. When low-pitched, this murmur is associated with pulmonary valve insufficiency in the absence of pulmonary hypertension. A low-pitched decrescendo diastolic murmur is typically noted after surgical repair of the pulmonary outflow tract in defects such as tetralogy of Fallot or in patients with absent pulmonary valves. A rumbling mid-diastolic murmur at the left middle and lower sternal border may be due to increased blood flow across the tricuspid valve, such as occurs with an atrial septal defect or, less often, because of actual stenosis of this valve. When this murmur is heard at the apex, it is caused by increased flow across the mitral valve, such as occurs with large left-to-right shunts at the ventricular level (ventricular septal defects), at the great vessel level (patent ductus arteriosus, aortopulmonary shunts), or with increased flow because of mitral insufficiency. When an apical diastolic rumbling murmur is longer and is accentuated at the end of diastole (presystolic), it usually indicates anatomic mitral valve stenosis. The absence of a precordial murmur does not rule out significant congenital or acquired heart disease. Congenital heart defects, some of which are ductal dependent, may not demonstrate a murmur if the ductus arteriosus closes. These lesions include pulmonary or tricuspid valve atresia and transposition of the great arteries. Murmurs may seem insignificant in patients with severe aortic stenosis, atrial septal defects, anomalous pulmonary venous return, atrioventricular septal defects, coarctation of the aorta, or anomalous insertion of a coronary artery. Careful attention to other components of the physical examination (growth failure, cyanosis, peripheral pulses, precordial impulse, heart sounds) increases the index of suspicion of congenital heart defects in these cases. In contrast, loud murmurs may be present in the absence of structural heart disease, for example, in patients with a large noncardiac arteriovenous malformation, myocarditis, severe anemia, or hypertension. Many murmurs are not associated with significant hemodynamic abnormalities. These murmurs are referred to as functional, normal, insignificant, or innocent (the preferred term). During routine random auscultation, more than 30% of children may have an innocent murmur at one time in their lives; this percentage increases when auscultation is carried out under nonbasal circumstances (high cardiac output because of fever, infection, anxiety). The most common innocent murmur is a medium-pitched, vibratory or “musical,” relatively short systolic ejection murmur, which is heard best along the left lower and midsternal border and has no significant radiation to the apex, base, or back. It is heard most frequently in children between 3 and 7 yr of age. The intensity of the murmur often changes with respiration and position and may be attenuated in the sitting or prone position. Innocent pulmonic murmurs are also common in children and adolescents and originate from normal turbulence during ejection into the pulmonary artery. They are higher pitched, blowing, brief early systolic murmurs of grade I–II in intensity and are best detected in the 2nd left parasternal space with the patient in the supine position. Features suggestive of heart disease include murmurs that are pansystolic, grade III or higher, harsh, located at the left upper sternal border, and associated with an early or midsystolic click or an abnormal 2nd heart sound. A venous hum is another example of a common innocent murmur heard during childhood. Such hums are produced by turbulence of blood in the jugular venous system; they have no pathologic significance and may be heard in the neck or anterior portion of the upper part of the chest. A venous hum consists of a soft humming sound heard in both systole and diastole; it can be exaggerated or made to disappear by varying the position of the head, or it can be decreased by lightly compressing the jugular venous system in the neck. These simple maneuvers are sufficient to differentiate a venous hum from the murmurs produced by organic cardiovascular disease, particularly a patent ductus arteriosus. The lack of significance of an innocent murmur should be discussed with the child's parents. It is important to offer complete reassurance because lingering doubts about the importance of a cardiac murmur may have profound effects on child-rearing practices, most often in the form of overprotectiveness. An underlying fear that a cardiac abnormality is present may negatively affect a child's self-image and subtly influence personality development. The physician should explain that an innocent murmur is simply a “noise” and does not indicate the presence of a significant cardiac defect. When asked, “Will it go away?” the best response is to state that because the murmur has no meaning, it does not matter whether it “goes away” or not. Parents should be warned that the intensity of the murmur might increase during febrile illnesses. However, with growth, innocent murmurs are less well heard and often disappear completely. At times, additional studies may be indicated to rule out a congenital heart defect, but “routine” electrocardiographic, chest roentgenographic, and echocardiographic examinations should be avoided in well children with innocent murmurs. Heart at the newborn is rather great, right and a left ventricle are rather peer. Auricles and the main vessels have a little big sizes in comparison with ventricles. The augmentation of mass and heart volume most intensively occurs in the first 2 years of a life and teenage age - from 12 till 14 years, and also from 17 till 20 years. Heart departments are enlarged irregularly, more intensively till 2 years auricles, with 2 till 10 years - all heart in whole grow, after 10 years ventricles are enlarged mainly. Till 6 years the form of heart the spherical. To 2 - 3 years heart is located horizontally. Borders of heart at children compare to age norms on groups: till 2 years, from 2 till 7 years, with 7 till 12 years. The myocardium at the newborn represents an undifferentiated sincytium. Muscular fibers thin, are weakly delimited from each other. Are weakly expressed longitudinal and cross-section striation. Till 2 years there is a reduction of muscular fibers and augmentation of diameter of spending monocytes. To 7 - to 8 years there is a definitive fabric differentiation of heart. To 14 to 15 years development of histological structures of spending system of heart comes to an end. To 4-year-old age warm activity is regulated basically by sympathetic nervous system to what the physiological tachycardia at children of the first years of a life is partially bound. Under the influence of a vagus nerve is rare a warm rhythm also can appear, elongation of intervals between warm reductions. Blood vessels of newborns thin-walled, muscular and elastic fibers in them are developed insufficiently. With the years at children systolic pressure grows, diastolic tends only to rising. By 12 years structure of vessels same, as at adults. Anatomically heart of the newborn is located more than at children of advanced age that is partially caused by higher standing of a diaphragm. The big axis of heart lays almost horizontally. The form of heart the spherical. In process of growth of the child (thoracal age) there is a heart turn from right to left and downwards, that leads to gradual change of borders of heart. Borders of relative warm dullness at a percussion Border Age of children in years Upper edge 0-1 II rib The left external 1 – 2 sm edge Right edge 2-6 The middlerib second 1 – 2 sm from the left From the left papillary line papillary line 7 - 12 III rib On a papillary line The right It is a little The distance Diameter of area of an parasternal line inside from a middle between the right obtusion, sm parasternal line parasternal line and a breast bone right edge 6-9 8 - 12 9 - 14 Blood vessels of newborns thin-walled, in them are insufficiently developed muscular and elastic fibers. The lumen of arteries is rather wide. The relation of a lumen of veins and arteries approximately 1: 1. As veins grow faster arteries by 16 years their lumen becomes twice wider than a lumen of arteries. To growth of arteries there is a development in them of a muscular cover and connective tissue elements. Transition walking changes hemodynamic conditions, promoting more intensive development of venous system of the bottom half of body. Along with the general pattern of growth of vessels of the big circle of a circulation the return picture occurs in arterioles of a small circle of a circulation: there is an involution of a muscular layer to an involution of walls of vessels. Pulse of the newborn is arrhythmic, and at children of all age more often, than at adults. Pulse rate at children Age Average frequency 2σ Newborns 1 - 12 months 2 - 6 years 7 - 12 years 140 130 - 115 110 - 105 95 - 85 50 45 35 – 40 30 Children's pulse is very much labial, and more objective data it is possible to receive in the morning, before transition of the child in vertical position. Arterial pressure at children depending on age Systolic BP Mm hg Age The newborn 1 year 5 years 10 years 15 years Diastolic BP Mm hg 60 80 – 84 100 110 120 makes ½ or 1/3 Systolic at all age The technique of inspection of the patient includes complaints, the anamnesis, a palpation, a percussion, auscultation, tool methods of research. Tool methods of research include heart roentgenography, an electrocardiogram. The semeiology of a lesion of heart and vessels at children is diverse. It is necessary to consider presence of a cyanosis, pallor of a skin, pains in heart and extremities, palpitation, a dyspnea, pulse change, augmentation of borders of heart, presence of warm hums. Syndromes of the basic lesions of heart include rhythm disturbances, myocardites, endocarditises, the pericardites got and congenital heart diseases, a heart failure syndrome.