How do Catalysts Work? - Lab

HOW DO CATALYSTS WORK?
INTRODUCTION
Hydrogen peroxide (H
2
O
2
) is a highly active chemical often used for cleaning minor wounds. Because it is toxic to cells, hydrogen peroxide will kill the cells if not immediately removed or broken down.
In this activity you will observe the effect of two substances which break down hydrogen peroxide. One of the substances, manganese dioxide, is an inorganic catalyst. A catalyst is a chemical that speeds the rate of a chemical reaction without being consumed in the reaction. The other substance, catalase, is an enzyme found in liver. An enzyme is a protein that acts as an organic catalyst.
EQUIPMENT
2 test tubes masking tape metric ruler hydrogen peroxide fine sand manganese dioxide
250 mL beaker hot plate
10 mL graduated cylinder test tube tongs forceps liver figure 1 mortar and pestle
PROCEDURE
Test 1 1. Label one test tube “A”.
2. Use tape to mark a line 2 cm from the bottom of the test tube.
3. Pour water up to the tape mark.
4. Add a pinch of fine sand to the tube.
5. What happened? Record the results.
Test 2 1. Label the other test tube “B”.
2. Repeat test 1 but use hydrogen peroxide instead of water.
Test 3 1. Add a pinch of manganese dioxide to test tube “A”.
2. What happened? Record the results.
Test 4 Repeat test 3 with test tube “B”.
Test 5 1. Boil test tube “A” in a water bath (see figure 1).
2. Add 5 mL of hydrogen peroxide.
3. What happened? Record the results.
Test 6 1. Clean out test tube “A”.
2. Pour hydrogen peroxide up to the tape mark.
3. Add a pinch of fine sand.
4. Use forceps to drop a small piece of liver into the test tube.
5. What happened? Record the results.
Test 7 1. Clean out test tube “B”.
2. Pour hydrogen peroxide up to the tape mark.
3. Add a pinch of fine sand.
4. Cut another small piece of liver.
5. Grind the liver in the mortar with 2 mL water and a pinch of sand.
6. Pour the ground liver into the test tube.
7. What happened? Record the results.
Test 8 1. Clean out test tube “A”.
2. Pour hydrogen peroxide up to the tape mark.
3. Add a pinch of fine sand.
4. Cut another small piece of liver.
5. Boil the liver in a beaker of water for about 5 minutes.
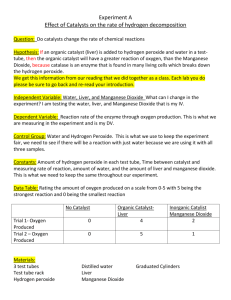
DATA
6. Use forceps to drop the liver into the test tube.
7. What happened? Record the results.
Rate the speed of the reaction: F = fast, S = slow, or N = none
_____ test 1: water + fine sand
_____ test 2: hydrogen peroxide + fine sand
_____ test 3: water + fine sand + manganese dioxide
_____ test 4: hydrogen peroxide + fine sand + manganese dioxide
_____ test 5: boiled water + fine sand + manganese dioxide + hydrogen peroxide
_____ test 6: hydrogen peroxide + fine sand + piece of liver
_____ test 7: hydrogen peroxide + fine sand + ground liver
_____ test 8: hydrogen peroxide + fine sand + boiled liver
ANALYSIS
1. What was the purpose of adding manganese dioxide to water in test 3?
_____________________________________________________________________
2. Do you think that manganese dioxide is consumed in the reaction?
______________ Explain. ______________________________________________
3. What steps could you take to confirm your answer to question #2?
_____________________________________________________________________
4. Consider the molecular formula of hydrogen peroxide: H peroxide?
2
O
2
What do you think are the products of the breakdown of hydrogen
______________ and ______________
5. What steps could you take to confirm your answer to question #4?
_____________________________________________________________________
6. What substance sped the reaction in tests 6 and 7?
___________________________
7. Why did the reaction rate differ between whole and ground liver?
_____________________________________________________________________
8. Why is test 2 necessary for your answer to question #7?
_____________________________________________________________________
9. Why did the reaction rate differ between ground and boiled liver?
_____________________________________________________________________
10. Suppose that someone compared the results of tests 4, 6, and 7 and concluded that liver contained manganese dioxide. Are they correct?
____________Explain_________________________________________________
_____________________________________________________________________