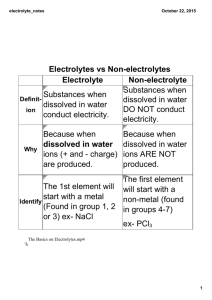
Low-temperature co-sintering of co-ionic conducting solid oxide fuel
advertisement

Low-temperature co-sintering of co-ionic conducting solid oxide fuel cells based on Ce0.8Sm0.2O1.9-BaCe0.8Sm0.2O2.9 composite electrolyte Dong Tiana,b, Wei Liua,*, Yonghong Chenb, Qinwen Gub, Bin Linb,* a CAS Key Laboratory of Materials for Energy Conversion, Department of Materials Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, P.R. China b Anhui Key Laboratory of Low temperature Co-fired Material, Huainan Normal University, Huainan 232001, P.R. China [*] Corresponding Author: Bin Lin* Anhui Key Laboratory of Low Temperature Co-fired Materials, Department of Chemistry, Huainan Normal University, Huainan, Anhui, 232001, P.R. China (tel) +86-554-6863553; (fax) +86-554-6863553; (E-mail): bin@mail.ustc.edu.cn (B. Lin) _____________ * Corresponding author. E-mail address: wliu@ustc.edu.cn (W. Liu); bin@mail.ustc.edu.cn (B. Lin). In order to confirm the absence of cation migration across the interfacial region, an EDS microanalysis has been carried out on the interface between anode and electrolyte. We can see from the figures as following, there is no migration of Ba2+ or Ni2+ ion. Fig SI1. An EDS microanalysis on the interface between anode and electrolyte. A comparison of the electrochemical performances of the synthesized materials in this work with others reported in literatures have been presented, which are shown as follows. We can seen from the Table SI1 that the single cell based on SDC-BSC composite electrolyte has an excellent electrochemical performances, especially at low operating temperature. Tabel SI1 Electrolyte, operating temperature, OCV, and peak power density reported in literature for SOFCs electrolyte Operating OCV Peak power Temperature (V) density references (mWcm−2 ) (℃) BaCe0.8Sm0.2O3-δ 700 0.96 160 1 BaCe0.8Sm0.2O3-δ 700 0.98 449 2 BaCeO3–BaCe0.8Sm0.2O3−δ 700 0.96 416 3 Ce0.8Sm0.2O3-δ 700 0.76 1010 4 Ce0.8Sm0.2O3-δ 650 0.8 820 4 SDC-BCS 650 0.77 621 This work SDC-BCS 550 0.85 381 This work Reference: 1. Wang Y, Wang H, Liu T, Chen FL, Xia CR. Improving the chemical stability of BaCe0.8Sm0.2O3-δ electrolyte by Cl doping for proton-conducting solid oxide fuel cell. Electrochem Commun. 2013;28:87-90. 2. Nian Q, Zhao L, He BB, Lin B, Peng RR, Meng GY, Liu XQ. Layered SmBaCuCoO5+δ and SmBaCuFe5+δ perovskite oxides as cathode materials for proton-conducting SOFCs. J Alloys Compounds. 2010; 492: 291-294. 3. Park I, Kim J, Lee H, Park J, Shin DW. BaCeO3-BaCe0.8Sm0.2O3-δ bi-layer electrolyte-based protonic ceramic fuel cell. Solid State Ionics, 2013; 252: 152-156. 4. Yang W, Hong T, Li S, M AH, Sun CW, Xia CR. Chen LQ. Perovskite Sr1-xCexCoO3-δ (0.05 ≤ x ≤ 0.15) as superior cathodes for intermediate temperature solid oxide fuel cells. Appl Mater Interfaces. 2013; 5: 1143-1148.