Requirements for electrolyte and refilling water for lead
advertisement

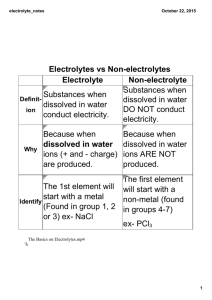
ZVEI information leaflet No. 3e Edition December 2011 Requirements for electrolyte and refilling water for lead acid batteries 1. General information This leaflet contains requirements for electrolyte and refilling water for lead acid batteries. The electrolyte for lead-acid accumulators is diluted sulfuric acid with density values related to type of construction of the accumulator or as specified by the battery manufacturer. Diluted sulfuric acid is used as filling acid for unfilled dry charged cells or batteries. Purified water used is for the preparation of diluted sulfuric acid and for refilling of cells or batteries. The purity of refilling water has to meet higher requirements than for filling electrolyte, because the impurities of the operating electrolyte will be increased by regular refilling of water. Electrolyte and water, not according to the requirements result in damages of the battery. mixing concentrated sulfuric acid or sulfuric acid with high density of d > 1.30 kg/l and purified water. Sulfuric acid in concentrated form is colorless, high etching liquid with a density d = 1.84 kg/l. 2.2. Water and refilling water Water (H2O) is an ingredient of the electrolyte for accumulators. Purified water is used for the preparation of electrolyte for accumulators and for refilling of water loss caused by overcharging and evaporation in the operating electrolyte. 2.3. Filling electrolyte Diluted sulfuric acid used for first filling of accumulators or for replacement of electrolyte in the case of contamination of the operating electrolyte is called filling electrolyte. 2.4. First filling The first filling denotes the filling with filling electrolyte which is generally carried out by the battery manufacturer or the user in accordance with the applicable manufacturer’s instructions for start-up. 2. Definitions 2.1. Electrolyte The electrolyte for lead-acid accumulators is diluted sulfuric acid ( H2SO4) with density values as specified by the battery manufacturer. Diluted sulfuric acid is prepared by 2.5. Operating electrolyte The operating electrolyte denotes the electrolyte which is used during operation of the battery. The density values and the degree of purity of the operating electrolyte may This leaflet was prepared by the Working Group Industrial Batteries of the ZVEI – German Electrical and Electronic Manufacturer’s Association deviate from the values of the filling electrolyte. 2.6. Electrolyte density The electrolyte density in kg/l denotes a measurement for the mass included in one unit volume. 2.7. Nominal density The nominal density of the electrolyte denotes a value specified by the battery manufacturer related to the nominal temperature, nominal electrolyte level and the fully charged state of the accumulator. In the case of lead-acid accumulators the density is a function of the state of charge. Density of the electrolyte for accumulators related to analytical statements Density values which are measured at temperatures deviating from the nominal temperature are converted to the nominal temperature of 25°C for the analytical evaluation. Analytical evaluation denotes information for the analysis of electrolytes. Density of electrolyte for accumulators related to the use and type of construction The nominal density of electrolyte related to the use and type of construction of the accumulator is specified by the manufacturer. Measurement of the electrolyte density As a rule, the measurement of the electrolyte density is carried out by densimeters, the socalled siphon acidimeters, in which areometers (hydrometers) are used. Electrolyte level The electrolyte level denotes the level of electrolyte in the accumulator. The permissible zone is indicated by electrolyte level marks (minimum, maximum) 2.8. Nominal temperature The nominal temperature is a specified value, used as a reference of important properties, such as the nominal electrolyte density and the nominal capacity of the accumulators. 2.9. Electrolyte additives Additives which are designated as agents of improvement are not permissible as they may cause damage to the battery in the longer term and thus endanger the functional safety. 2.10. Impurities In practical use, the electrolyte can be contaminated which may cause damage to the accumulator or reduce its power. The type and maximum permissible quantity of impurities are specified in the following tables. 3. Water use for topping up and preparation of electrolyte The water shall meet the physical requirements as shown in Table 1 and the chemical requirements as given in Table 2. Purified water in compliance with the require-ments can be prepared from tap water by distillation or by ion exchange. 3.1. Physical requirements of purified water Appearance clear, colorless, odorless, no oil drips pH value 5 to 7 electric conductivity at 20°C - freshly prepared - up to being filled into the cell 4. Storage of purified water Water shall be stored in appropriate vessels, such as vessels made of glass, ebonite, polyethylene, polypropylene or other plastic materials. Hoses should be made of PVC, rubber or polyethylene. The dissolution of metal ions out of metallic vessels is liable to occur. Vessels made of metal, shall therefore, not be used. It is recommended that purified water should always be stored in airtight vessels because carbon dioxide (CO2) which is absorbed from the air, is increasing the electric conductivity of water. < 10 μS/cm < 30 μS/cm Table 1 3.2. Chemical requirements of purified water The purified water shall not exceed the limit values given in Table 2. No. Impurities mg/l max. 1 evaporation residue 10 2 oxidable organic substances calculated as KMnO4 20 3 metals of the hydrogen sulfide group (Pb, Sb, As, Sn, Bi, Cu, Cd ) each element individually all together 0.1 0.5 4 Metals of the ammonium sulfide group (Fe, Co, Ni, Cu, Cr) each element individually all together 0.1 0.5 5 Halogens calculated as chloride 0.5 6 Nitrogen as nitrate 2.0 7 Nitrogen as e.g. Ammonia 40 Table 2 2/5 5. Preparation of electrolyte for lead-acid accumulators The electrolyte is prepared from sulfuric acid of high concentration by pouring it into purified water in accordance to point 3. Note: - The electrolyte shall only be prepared by the battery manufacturer or skilled personnel. - Concentrated and diluted sulfuric acid has a highly etching effect on human skin, clothes and the appliance. - Never add water by pouring it into concentrated acid - Note the safety data sheets. 6. Physical properties of diluted sulfuric acid as electrolyte 6.2. Correction of density from measuring temperature to nominal temperature specific gravity dN kg/l correction factor fd*) kg/l per K 1.10 0.00050 1.15 0.00060 1.20 0.00070 1.30 0.00075 *) The correction factor refers to the temperature range from 0°C to 55°C Table 3 6.3. Dependence of the specific gravity on the contents of sulphuric acid at 25°C specific gravity at 25°C kg/l 1.100 % mol/l mass concentration g/l 15.18 1.704 166.98 1.110 16.45 1.863 182.60 1.120 17.80 2.034 199.36 1.130 19.15 2.208 216.40 1.140 20.47 2.381 233.36 1.150 21.81 2.558 250.70 dn = dT + fd (T – Tn) 1.160 23.11 2.735 268.07 meaning 1.170 24.39 2.911 285.36 1.180 25.63 3.086 302.43 1.190 26.90 3.266 320.11 6.1. Dependence of specific gravity on temperature The acid densities which occur at the measuring temperature and deviate from the nominal density shall be converted to the acid densities at nominal temperatures. dn specific gravity at nominal temperature Tn mass portion amount of substances 1.200 28.12 3.443 337.44 dT specific gravity at measuring temperature T 1.210 29.34 3.622 355.01 1.220 30.55 3.803 372.71 fd correction factor according to Table 3 1.230 31.78 3.989 390.89 1.240 32.98 4.173 408.95 T measuring temperature 1.250 34.18 4.360 427.25 1.260 35.40 4.551 446.04 1.270 36.60 4.743 464.82 1.280 37.81 4.938 483.97 1.290 38.93 5.124 502.20 1.300 40.10 5.319 521.30 Tn nominal temperature Table 4 6.4. Dependence of the specific gravity on the state of discharge The specific gravity decreases during discharge of an accumulator, the permissible limit values are given by the battery manufacturer for the various applications. 3/5 7. Requirements of sulfuric acid used as electrolyte 7.1. Impurities of sulfuric acid of higher concentration degrees The purity of higher concentrated sulfuric acid shall be so, that after the dilution with water to filling electrolyte densities of < 1.30 kg/l the values specified in Table 5 are not exceeded. Impurities mg/l max. 1 metals of platinum group 0.05 2 rhenium 0.1 3 copper 0.5 4 other metals of the hydrogen sulfide group other than lead, e.g. arsenic, antimony, bismuth, tin, selenium, tellurium individually all together 1.0 2.0 5 manganese, chromium, titanium, nickel individually 0.2 6 iron 30 7 other metals of the ammonium sulfide group other than aluminum and zinc, e.g. cobalt individually all together 1.0 2.0 Cons No. 8 halogens calculated as chloride 5 9 nitrogen as nitrate 10 10 nitrogen as e.g. ammonia 50 11 volatile organic acids calculated as acetic acid 20 12 oxidable organic substances calculated as KMnO4 consumption 30 13 annealing residue 250 Table 5 - Permissible impurities of diluted sulfuric acid as filling electrolyte for lead-acid batteries in the density range ≤ 1.30 kg/l 7.2. Impurities of filling acid and operating acid The sulfuric acid used for filling lead-acid batteries shall be clear and colorless. The impurities included in the acid shall not exceed any value quoted in Table 5. For operating electrolyte, the maximum values of Table 6 shall apply. 2) Impurities mg/l max. 1 metals of platinum group n.m. 1) 2 rhenium n.m. 1) 3 copper n.m. 1) 4 tellurium and selenium individually 5 other metals of the hydrogen sulfide group other than lead and antimony, e.g. arsenic, bismuth individually all together 3 6 6 Antimony a) stationary cells with Plantè plates or flat plates b) stationary cells with tubular plates and traction cells 3 10 7 manganese, chromium, titanium, nickel individually 0.2 8 iron 100 9 other metals of the ammonium sulfide group other than aluminum and zinc e.g. cobalt individually all together 1.0 2.0 10 halogens calculated as chloride a) stationary cells b) traction cells 50 500 11 nitrogen in the form of nitrate 10 12 nitrogen in other form e.g. ammonia 50 13 volatile organic acids calculated as acetic acid 30 14 oxidable organic substances calculated as KMnO4 consumption 50 Cons No. 1.0 Table 6 - Permissible impurity of diluted acid and operating acid for lead-acid batteries in the density range ≤ 1.30 kg/l 1) nm = not measurable / These metals remain deposited virtually completely on the negative electrode. These harmful substances effect a high self-discharge 4/5 2) It is not possible to specify valid limit values for metals in general. The levels of impurities which are harmful to the batteries depend strongly on other parameters as type, age and operating conditions of the cell. 8. Storage of electrolyte Electrolyte which is intended to be stored shall be placed in vessels which are appropriately marked and shall be resistant to chemical corrosion (e.g. polyethylene, polypropylene or similar plastic material). 9. Remedy in the event of damage due to electrolyte 10. Additional instructions for safe handling Where parts of skin, eyes or mucous membranes have been exposed to the harmful effect of the electrolyte, immediate actions shall be taken in all these cases by rinsing the affected parts with plenty of water. Additionally medical care is required. For additional instructions refer to ZVEI Information leaflets • Instructions for safe handling of lead-acid accumulators (leadacid batteries) • Safety data sheet on accumulator acid (diluted sulphuric acid) Appliances, installations and clothes may be cleaned by using neutralization agents and by rinsing them out with water. Soda solutions of a concentration of 5% (sodium carbonate) or solid soda can be used in order to reduce the affect of electrolyte on appliances, the human body and clothes. Diatomaceous earth is especially suited absorbing spilled electrolyte. Editor: ZVEI – Zentralverband Elektrotechnik- und Elektronikindustrie e. V. Fachverband Batterien Lyoner Straße 9 60528 Frankfurt Fon.: +49 69 6302-283 Fax: +49 69 6302-362 Mail: batterien@zvei.org www.zvei.org © ZVEI 2011 In spite of all due care, however, we cannot accept any liability that the information is complete or correct or up to date. 5/5