How to make Molecular Biology Reagents
advertisement

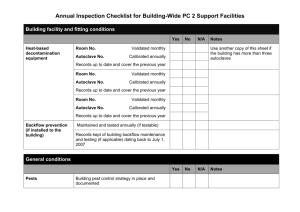
Dr. Bagga Lab Protocols Cell & Molecular Biology SBIO 406 Experiment 1 HOW TO MAKE MOLECULAR BIOLOGY REAGENTS A scientist is as good as the techniques he/she can perform successfully. The success of the technique depends on the experimental design, precision and quality of the reagents used. A molecular biologist, since works at molecular level, has to be very careful with the quality of the reagents. Even a very low level of contamination can either ruin an experiment or lead to erroneous results. On the other hand even a slight variation in the concentration or pH of an ingredient of a reagent can negatively affect the success of the experiment. The training of a molecular biologist, therefore, has to start with learning to make quality reagents. Here are some important tips to keep in mind before making a reagent for molecular biology experiments: Start with accurate calculation (on a paper) the amounts of chemicals to be measured. Use a well calibrated weighing balance to weigh the solids. Pipetters should be calibrated periodically. Use clean, fresh, spatula for each different chemical to be weighed. Never put the chemical back into the container All the glassware and containers including beakers, flasks, bottles, test tubes, eppendorf tubes, pipette tips etc. should be autoclaved before use, unless the reagent stored in it will be autoclaved within an hour or two. No need to autoclave sterile disposables. Read about dissolution and other properties of each chemical before handling them. Always use double-distilled glass distilled water. Never put the entire water (or other solvent) to dissolve the chemical initially. Volumes should be made up only after complete dissolution and pH adjustment (if required). Clean the pH electrode with distilled water before and after the to prevent cross contamination. Use appropriate measuring cylinder for making up the volumes. Most solutions need to be sterilized by autoclaving. Heat sensitive solutions should be prepared in autoclaved distilled water and then filter sterilized. It is a good practice to make concentrated stock solutions and distribute them in convenient aliquots. The actual reagents can be made (fresh when needed) by mixing calculated aliquots from the stock solutions. Store the reagents appropriately on the shelf, refrigerator or freezer. Watch out for microorganism growth. When in doubt, discard the reagent. Never put the solution back into the stock container. Always use a fresh, sterile pipette or pipette tip for each dispensing. Always use gloves for safety as well as to prevent contamination. Fingertips are a common source of protease and nuclease contaminations. Observe Lab Safety rules. Always wear Lab coat. Use protective glasses and fume hood when necessary. You will be given more detailed instructions in the laboratory 1 Dr. Bagga Lab Protocols Cell & Molecular Biology SBIO 406 Materials/ Supplies for the Class 2 pH meters, Standard Buffers, 10 N Sodium Hydroxide, 6 N HCl 2 Magnetic Stirrers 2 Weighing Balances, Plastic boats for weighing (various sizes) 10 Spatulas Gloves 2 Wash Bottles with D.W. 2 x 150 ml Beakers 2 liters of distilled water Kimwipes Autoclave Note: This lab will be divided among four groups of students. Each group should consist of four students maximum. Group 1 Objective: To prepare 250 ml of 0.5 M EDTA (pH 8.0) Materials: Micropipettors, tips (autoclaved) Pipettors (5-10 ml and 1-2 ml) and pipettes (1 ml, 5 ml and 10 ml) Micro Eppendorf tubes (Autoclaved) Permanent Marker 2 x 500 ml Beakers 250 ml Beaker Magnetic stir bar Magnetic stir bar remover Magnetic Stirrer 500 ml Screwcap autoclave bottle 250 ml Graduated cylinder 50 ml Polypropylene screw capped tube in a rack EDTA Sodium Salt NaOH Pellets Instructions 1. Calculate the amounts of EDTA to be weighed in order to prepare 250 ml of 500 mM solution. 2. Weigh the calculated amount of EDTA and pour it in ~ 150 ml of D.W. in a 500 ml beaker. Use magnetic stirrer and stir bar to assist dissolution. 3. Lower a cleaned electrode of the pH meter into the solution. 4. You will notice that pH will start to go down following dissolution of EDTA. This will make dissolution difficult because EDTA dissolves at neutral to alkaline pH. 5. You would need to gradually add NaOH PELLETS to the solution to maintain pH close to 8.0. Caution! Don’t let pH go too much higher than 8.0. 6. After the entire EDTA powder is dissolved, adjust the final volume to 250 ml. 2 Dr. Bagga Lab Protocols Cell & Molecular Biology SBIO 406 7. Adjust pH if necessary with 10 N NaOH or 6 N HCl to 8.0. 8. Dispense the EDTA solution in appropriately labeled glass bottle and autoclave at a pressure of 15 lb/in2 (121oC) for 20 minutes. Remember to leave the bottle cap loose before putting them in the autoclave. 9. Remove the bottle after the pressure has been released from the autoclave (Careful, Hot liquids !!!). 10. Tighten the cap only after the liquids cool down. Store in the refrigerator. Group 2 Objectives: A. To prepare 250 ml stock solution of 1 M sodium chloride B. To prepare 250 ml stock solution of 1 M Tris.HCl buffer (pH 6.8) Materials: Micropipettors, tips (autoclaved) Pipettors (5-10 ml and 1-2 ml) and pipettes (1 ml, 5 ml and 10 ml) Micro Eppendorf tubes (Autoclaved) Permanent Marker 3 x 500 ml Beakers 2 x 250 ml Beakers Magnetic stir bar Magnetic stir bar remover Hot plate Magnetic Stirrer 2 x 500 ml Screwcap autoclave bottles 2 x 250 ml Graduated cylinders 50 ml Polypropylene screw capped tube in a rack Sodium Chloride (Powder) Trizma Base (Powder) Instructions 1. Calculate the amount of sodium chloride and Trizma base to be weighed. 2. Weigh and dissolve the calculated amount of sodium chloride in 200 ml of D.W. Use magnetic stirrer and stir bar to assist dissolution. Heat might be necessary to dissolve the salt. 3. Adjust the final volume to 250 ml. 4. Weigh the appropriate amount of Trizma base and dissolve it in 150 ml of D.W. Use magnetic stirrer and stir bar to assist dissolution. 5. Adjut the pH to 6.8 with 6 N HCl 6. Adjust the final volume to 250 ml. 7. Dispense both solutions in appropriately labeled glass bottles and autoclave at a pressure of 15 lb/in2 (121oC) for 20 minutes. Remember to leave the bottle caps loose before putting them in the autoclave. 8. Remove the bottles after the pressure has been released from the autoclave (Careful, Hot liquids !!!). 9. Tighten the caps only after the liquids cool down. Store at room temperature. 3 Dr. Bagga Lab Protocols Cell & Molecular Biology SBIO 406 Group 3 Objectives: A. To prepare a 50 ml stock solution of 10% SDS B. To prepare a 5 ml stock solution of 1% Bromophenol Blue Materials: Micropipettors, tips (autoclaved) Pipettors (5-10 ml and 1-2 ml) and pipettes (1 ml, 5 ml and 10 ml) Micro Eppendorf tubes (Autoclaved) Sensitive balance to weigh milligram quantities. Permanent Marker 100 ml Beaker 250 ml Beaker Magnetic stir bar (Small) Magnetic stir bar remover Hot plate Magnetic Stirrer 100 ml Screwcap autoclave bottle 100 ml Graduated cylinder 15 ml blue cap tube in rack Sodium Dodecyl Sulfate (SDS) Powder Bromophenol Blue (Powder) Instructions 1. Calculate the amount of sodium dodecyl sulfate (SDS) and Bromophenol blue to be weighed. 2. Weigh the appropriate amount of SDS and dissolve it in 40 ml of D.W. Use magnetic stirrer and stir bar to assist dissolution. Avoid frothing. Heat might be necessary to dissolve the detergent. 2. Adjust the final volume to 50 ml. 3. Store in an appropriately labeled bottle at room temperature. 4. Weigh the appropriate amount of Bromophenol Blue and dissolve in 5 ml of D.W. 5. Store in labeled sterile polypropylene screw capped tube at room temperature Group 4 Objective: To prepare a 4 ml of 2x Protein Dye Buffer of following composition: 125 mM Tris buffer(pH 6.8) 20% Glycerol 4 % SDS 0.05 % Bromophenol Blue 10% beta mercaptoethanol Materials: Micropipettors, tips (autoclaved) Pipettors (5-10 ml and 1-2 ml) and pipettes (1 ml and 5 ml) 4 Dr. Bagga Lab Protocols Cell & Molecular Biology SBIO 406 Micro Eppendorf tubes (Autoclaved) Rack for Micro Eppendorf tubes 15 ml blue cap tube in a rack Permanent Marker Bucket of Ice Autoclaved Glycerol (50 ml) Beta Mercapto ethanol Instructions 1. Stock solutions to be used: 1M Tris.HCl (pH 6.8), Glycerol, 10 % SDS, 1% Bromophenol Blue and beta mercaptoethanol. 2. Stock solutions of 1M Tris.HCl (pH 6.8), 10 % SDS and 1% Bromophenol Blue can be obtained from other groups in the class. Beta mercaptoethanol can be used as such. You would need to autoclave (15 lb/in2 for 20 minutes) ~50 ml of glycerol in a bottle. Let it cool down before use. 3. Calculate the amounts of stock solutions and D.W. to be mixed together. 4. Use precision pipettors to mix the calculated volumes of stock solutions and D.W. in a 5 ml sterile test tube on ice. 5. Aliquot 0.5 ml in each of the eight appropriately labeled eppendorf test tubes (on ice). 6. Store at -200C. Calculation exercises for practice will be provided in the class 5