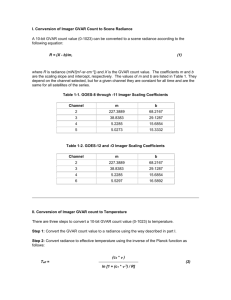
This is a fictitious sample Statement of Work intended to assist
advertisement
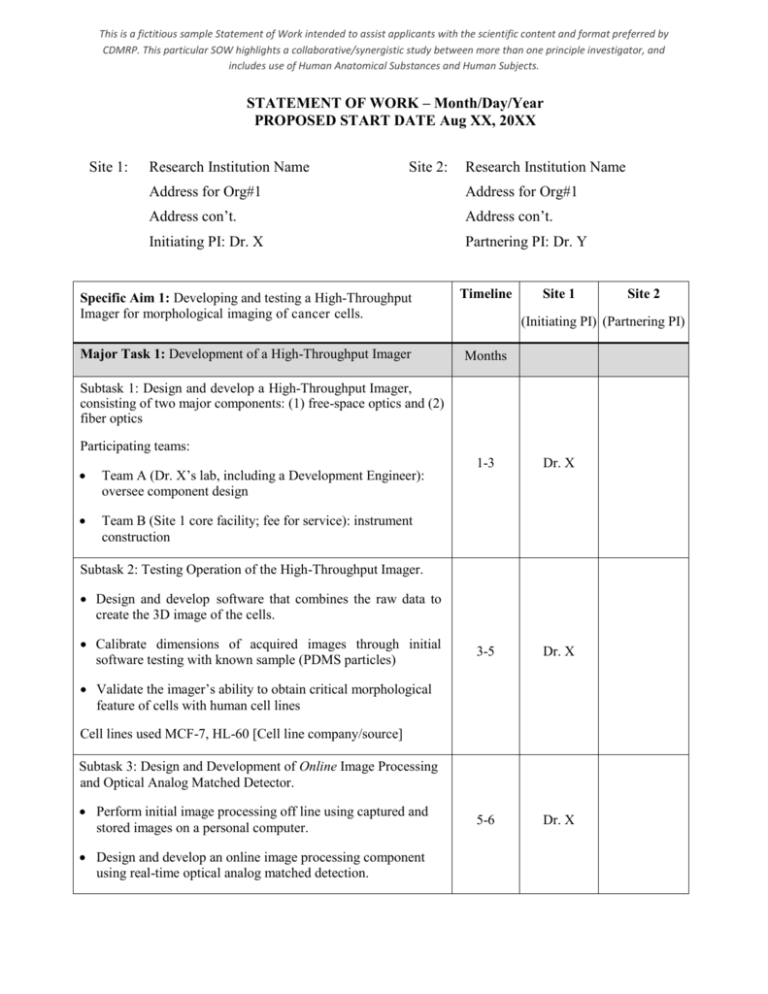
This is a fictitious sample Statement of Work intended to assist applicants with the scientific content and format preferred by CDMRP. This particular SOW highlights a collaborative/synergistic study between more than one principle investigator, and includes use of Human Anatomical Substances and Human Subjects. STATEMENT OF WORK – Month/Day/Year PROPOSED START DATE Aug XX, 20XX Site 1: Research Institution Name Site 2: Research Institution Name Address for Org#1 Address for Org#1 Address con’t. Address con’t. Initiating PI: Dr. X Partnering PI: Dr. Y Specific Aim 1: Developing and testing a High-Throughput Imager for morphological imaging of cancer cells. Timeline Major Task 1: Development of a High-Throughput Imager Months Site 1 (Initiating PI) (Partnering PI) Subtask 1: Design and develop a High-Throughput Imager, consisting of two major components: (1) free-space optics and (2) fiber optics Participating teams: Team A (Dr. X’s lab, including a Development Engineer): oversee component design Team B (Site 1 core facility; fee for service): instrument construction 1-3 Dr. X 3-5 Dr. X 5-6 Dr. X Subtask 2: Testing Operation of the High-Throughput Imager. Design and develop software that combines the raw data to create the 3D image of the cells. Calibrate dimensions of acquired images through initial software testing with known sample (PDMS particles) Validate the imager’s ability to obtain critical morphological feature of cells with human cell lines Cell lines used MCF-7, HL-60 [Cell line company/source] Subtask 3: Design and Development of Online Image Processing and Optical Analog Matched Detector. Perform initial image processing off line using captured and stored images on a personal computer. Design and develop an online image processing component using real-time optical analog matched detection. Site 2 This is a fictitious sample Statement of Work intended to assist applicants with the scientific content and format preferred by CDMRP. This particular SOW highlights a collaborative/synergistic study between more than one principle investigator, and includes use of Human Anatomical Substances and Human Subjects. Major Task 2: Imaging-based Detection of commercially available human cell lines. Subtask 1: Training of Dr. Y’s team by Dr. X’s staff on operation of High-Throughput Imager, including on-line image processing capability 6-7 Dr. X Subtask 2: Standardize the real-time optical analog matched detection. Dr. Y Dr. Y 7-9 Cell lines used MCF-7, SKOV3 and HL-60 [Cell line company/source] Milestone #1: Co-author manuscript on development of imager and initial studies. 6-12 Dr. X Dr. Y Subtask 1: Submit documents for local IRB* review. 1-3 Dr. X Dr. Y Subtask 2: Submit IRB approval and necessary documents for HRPO* review. 3-6 Dr. X Dr. Y Milestone #2: HRPO** approval received 6-9 Dr. X Dr. Y Specific Aim 2: Feasibility and Pre Clinical Studies using the High-Throughput Imager for morphological imaging of cancer cells. Major Task 3: Conduct initial feasibility studies to assess the ability to transfer to detection in human patients. Subtask 3: Preliminary study to verify detection of spiked cancer cells in peripheral blood Clinical validation of results with banked peripheral blood samples of confirmed cancer patients Dr. Y (N=100) 9-12 Human Anatomical Substances (HAS) used: deidentified peripheral blood samples [Univ X tissue bank] Subtask 4: Recruit, consent, and enroll 20 patients/human subjects to preclinical study. Conduct pre-clinical/ initial blinded study with patients whose diagnosis is matched to image results at end of study. 12-21 Dr. X (N=10) Dr. Y (N=10) This is a fictitious sample Statement of Work intended to assist applicants with the scientific content and format preferred by CDMRP. This particular SOW highlights a collaborative/synergistic study between more than one principle investigator, and includes use of Human Anatomical Substances and Human Subjects. Subtask 5: Decision on indicated uses of imager and pursuit of FDA approval path. 18-24 Dr. X Dr. Y 18-24 Dr. X Dr. Y Co-author manuscript on entire study. Milestone #3: Manuscript on use of the imager in pre-clinical studies. Projected Quarterly Enrollment Year 1 Target Enrollment Q1 Q2 Q3 Year 2 Q4 Q1 Q2 Q3 Site 1 3 4 3 Site 2 3 4 3 Target Enrollment 6 14 20 Q4 (per quarter) (cumulative) * IRB = Institutional Review Board; committee formally designated to approve, monitor, and review human subjects research ** HRPO = Human Research Protection Office; review and approval by HRPO office of protocols involving human subjects is required of all DoD-funded awards