• I will be able to
advertisement

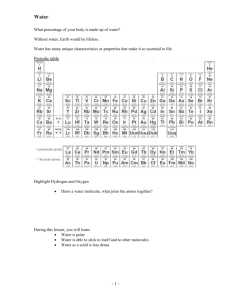
• I will be able to: • Describe the structure of water. • Describe the four main properties of water. • Describe Water’s role / importance on Earth. • Pick up handout and half sheet. • Copy homework. • Work on the heat transfer review. • Log on to laptop. • ES.0.7 Energy and Heat Transfer RHW Quiz due Tonight • ES. Intro Test Part 2 (ES.0.5-ES.0.7) Monday • ES.0.8 Properties of Water RHW Quiz due 10/5 I will be able to describe the structure of water and the properties of water. What is the Structure of Water? • Water Molecule – Made up of atoms. – Atoms join to form molecules – H20 • Hydrogen = ___ atoms • Oxygen = ___ atoms I will be able to describe the structure of water and the properties of water. What Makes Water a Polar Molecule? • Water Molecule – Polarity- anything that has one positive charge at one end and a negative charge at another. – Water is a Polar Molecule • Hydrogen= ____ Charge • Oxygen = ____ Charge – Negative atom is attracted to the positive atom. I will be able to describe the structure of water and the properties of water. I will be able to describe the structure of water and the properties of water. What are four properties of Water? • Polarity Affects: – Properties of water. – How water molecules interact with one another or other molecules. • • • • Cohesion Adhesion Specific Heat Solvents I will be able to describe the structure of water and the properties of water. What are four properties of Water? • Cohesion – A property that holds molecules together. – Water molecules are polar and are tightly held together. I will be able to describe the structure of water and the properties of water. What are four properties of Water? • Adhesion – A property that holds molecules of different substances together. – Other polar substances attract water molecules. – Wettable, water adheres or sticks.. I will be able to describe the structure of water and the properties of water. What are four properties of Water? • Specific Heat – Energy needed to heat a substance by a particular amount. – Water = High Specific Heat • Particles when heated: – Move faster – Spread apart. • Requires a lot of energy to separate the polar molecules. I will be able to describe the structure of water and the properties of water. What are four properties of Water? • Solvents – A liquid that dissolves substances. – Water = Universal Solvent • Dissolves many substances. • Solute- the substance dissolved. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Only planet with water in our solar system. – Exists in all 3 States and can change state. • Most is liquid I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Solid (ice / snow) (H2O) • Expands when frozen. • Less dense, floats in water. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Liquid- Rain, Water (H2O) • Precipitation • Gravity causes it to flow down hill. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Gas- Water Vapor (H2O) • Invisible in the atmosphere • Forms clouds. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – ___% Freshwater – _____ % of the freshwater is Frozen I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Reshapes the Surface • Water flows • Weathering = – Breaking up rock. • Erosion = – Movement – Influences Weather • • • • Water Cycle Clouds Precipitation Storms I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Supports Life • Cells = 70% water • Biological processes – Regulates temperature – Transports substances. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Need clean water to drink to survive. – Contaminated water is a major health problem. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – Water is a non-renewable resource. – Clean water is needed for: • Drinking • Bathing • Growing food. I will be able to describe the role and importance of water on Earth. What is Water’s Role on Earth? • Water on Earth – 20% Used for Industrial Processes. • Manufacturing • Agriculture • Generating Electricity – Cooling power stations – Hydroelectric dams • I will be able to: • Describe the structure of water. • Describe the four main properties of water. • Describe Water’s role / importance on Earth. • Copy homework. • Log on to Laptop, Take Sci Design Pre-Test in the ES.0.8 Folder • ES. Intro Test Part 2 (ES.0.5-ES.0.7) Monday • ES.0.8 Properties of Water RHW Quiz due 10/5 • I will be able to: • Describe the structure of water. • Describe the four main properties of water. • Describe Water’s role / importance on Earth. • Copy homework. • Log on to Laptop, Take Sci Design Pre-Test in the ES.0.8 Folder • ES. Intro Test Part 2 (ES.0.5-ES.0.7) Friday • ES.0.8 Properties of Water RHW Quiz due 10/5 I will be able to determine if a penny holds more Isopropyl Alcohol or freshwater. Cohesion / Adhesion Lab • Pre-Lab Questions – Determine the number of drops that can be placed on each penny (fresh water / Isopropyl Alcohol) – 1. Problem? • Will a penny hold more freshwater or Isopropyl Alcohol? I will be able to determine if a penny holds more Isopropyl Alcohol or freshwater. Cohesion / Adhesion Lab • Pre-Lab Questions – Determine the number of drops that can be placed on each penny (fresh water / Isopropyl Alcohol) – 12. Hypothesis? • ~If drops of fresh and alcohol are placed on a penny, then a penny will hold more drops of _________________than __________. – 3. Independent Variable – 4. Dependent Variable – 5. Control Group I will be able to determine if a penny holds more Isopropyl Alcohol or freshwater. Drops of Water on a Penny • Procedure: – Use the eyedropper (pipette) to drop fresh or Isopropyl alcohol onto the surface of the penny (heads side) drop by drop. – Count the number of drops that fit on the penny before the water spills off the penny. – Complete three trials. (switch off with your partner) – Calculate the average for all four trials. – Answer Analysis Questions. I will be able to describe the states of water that occur on Earth and the role of water. Diagram: • Identify: – Heat transfers: Conduction, Convection – Explain how convection works. I will be able to describe the states of water that occur on Earth and the role of water. Diagram: