Lec.1 Benzene
advertisement

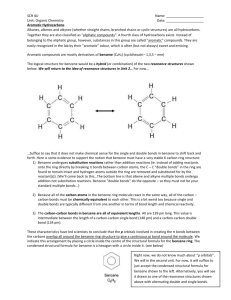
Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Aromatic Hydrocarbons:In the early part of the nineteenth century chemists began to discover organic compounds having chemical properties quite distinct from the alkanes, alkenes, and alkynes. They called these substances aromatic compounds because many of the first examples were isolated from the pleasant-smelling resins of tropical trees. The carbon: hydrogen ratio of these compounds suggested a very high degree of unsaturation, similar to the alkenes and alkynes. Imagine, then, how puzzled these early organic chemists must have been when they discovered that these compounds do not undergo the kinds of addition reactions common for the alkenes and alkynes. We no longer define aromatic compounds as those having a pleasant aroma; in fact, many do not. We now recognize aromatic hydrocarbons as those that exhibit a much higher degree of chemical stability than their chemical composition would predict. The most common group of aromatic compounds is based on the six member aromatic ring, the benzene ring. The structure of the benzene ring is represented in various ways in Figure 12.6. 1 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Structure and Properties:The benzene ring consists of six carbon atoms joined in a planar hexagonal arrangement. Each carbon atom is bonded to one hydrogen atom. Friedrich Kekulè proposed a model for the structure of benzene in 1865. He proposed that single and double bonds alternated around the ring (a conjugated system of double bonds). To explain why benzene did not decolorize bromine—in other words, didn’t react like an unsaturated compound—he suggested that the double and single bonds shift positions rapidly. We show this as a resonance model today. 2 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry The current model of the structure of benzene is based on the idea of overlapping orbitals. Each carbon is bonded to two others by sharing a pair of electrons(σ_bonds). Each carbon atom also shares a pair of electrons with a hydrogen atom. The remaining six electrons are located in p orbitals that are perpendicular to the plane of the ring. These p orbitals overlap laterally to form pi (╥) orbitals that form a cloud of electrons above and below the ring. These ╥_ orbitals are shaped like doughnuts, as shown in Figure 12.7. Two symbols are commonly used to represent the benzene ring. The representation in Figure 12.6b is the structure proposed by Kekulé. The structure in Figure 12.6d represents the╥ _ clouds. The equal sharing of the six electrons of the p orbitals results in a rigid, flat ring structure, in contrast to the relatively flexible, nonaromatic cyclohexane ring. The model also explains the unusual chemical stability of benzene and its resistance to addition reactions. The electrons of the╥ _ cloud are said to be delocalized. That means they have much more space and freedom of movement than they would have if they were restricted to individual double bonds. Because electrons repel one another, the system is more stable when the electrons have more space to occupy. As a result, 3 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry benzene is unusually stable and resists addition reactions typical of alkenes. Benzene Derivatives:The nomenclature of substituted benzene ring compounds is less systematic than that of the alkanes, alkenes and alkynes. A few mono-substituted compounds are named by using a group name as a prefix to "benzene", as shown by the combined names listed below. A majority of these compounds, however, are referred to by singular names that are unique. There is no simple alternative to memorization in mastering these names. 4 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Two commonly encountered substituent groups that incorporate a benzene ring are phenyl, abbreviated Ph-, and benzyl, abbreviated Bn-. These are shown here with examples of their use. Be careful not to confuse a phenyl (pronounced fenyl) group with the compound phenol (pronounced feenol). A general and useful generic notation that complements the use of R- for an alkyl group is Ar- for an aryl group (any aromatic ring). When more than one substituent is present on a benzene ring, the relative locations of the substituents must be designated by numbering the ring carbons or by some other notation. In the case of disubstituted benzenes, the prefixes ortho, meta & para are commonly used to indicate a 1,2- or 1,3- or 1,4- relationship respectively. In the 5 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry following examples, the first row of compounds show this usage in red. Some disubstituted toluenes have singular names (e.g. xylene, cresol & toluidine) and their isomers are normally designated by the ortho, meta or para prefix. A few disubstituted benzenes have singular names given to specific isomers (e.g. salicylic acid & resorcinol). Finally, if there are three or more substituent groups, the ring is numbered in such a way as to assign the substituents the lowest possible numbers, as illustrated by the last row of examples. The substituents are listed alphabetically in the final name. If the substitution is symmetrical (third example from the left) the numbering corresponds to the alphabetical order. 6 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Reaction of Benzene:1- Electrophilic Aromatic Halogenation:One of the substitutions that can occur with Benzene or one of its derivatives is to replace a Hydrogen atom on the ring with a halogen. 7 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Figure 1 above shows the net reaction for halogenation of a Benzene ring. The AlX3 acts as a Lewis acid catalyst. Chlorination and Bromination is possible using such a catalyst. Iodination requires the use of I-Cl as the Iodination reagent. Flourination is much too explosive to be a practical synthesis. The reaction mechanism (Fig 2) shows initially in the first step the generation of the Cl+ electrophile. The catalyst is required in step 1 because the halogen molecule is non-polar. The AlCl3 forces the bonding electrons in the Cl-Cl molecule to one end so that an induced polarity is effected. This weakens the co-valent bond between the two halogen atoms so that the bond breaks heterolytically generating the Cl + electrophile 8 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry 2- Electrophilic Aromatic Nitration Of Benzene Nitration of Benzene (Fig 1) or one of its derivatives involves a rather unusual nitrating mixture. Sulfuric Acid and Nitric Acid are mixed where the stroger Sulfuric Acid forces a proton on Nitric Acid. The first two steps in the Reaction Mechanism (Fig 2) shows how the nitro electrophile was generated. Notice that the Sulfuric Acid is regenerated and therefore serves as a catalyst in this reaction. The generation of a positively charged Oxygen atom (oxonium ion) in step 9 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry 1 produces an unstable intermediate which spontaneously decomposes losing water and generating the electrophile in step 2. 3- Electrophilic Aromatic Sulfonation of Benzene Aromatic Sulfonation places a Sulfonyl group (-SO3H) onto the Benzene ring. It accomplishes this by the use of Sulfur Trioxide in the presence of Sulfuric Acid. (Fig 1-a) Sulfur Trioxide gas is dissolved into concentrated Sulfuric Acid at 20% to give what is called "fuming Sulfuric Acid" which earns its name by billowing out white plumes of smokey SO3 fumes when the bottle is opened. The Sulfur Trioxide (SO 3) serves two purposes. It acts first as the sulfonating agent, and it prevents the unfavorable reversable of this equilibrium by reacting with and effectively removing the water product thus driving this equilibrium to the right. SO3 + H2O ---> H2SO4 The Sulfonation can be accomplished without the Sulfur Trioxide by using excess Sulfuric Acid (Fig 1-b). The extra Sulfuric Acid will act as a dehydrating agent 10 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry absorbing the water and preventing the reversal of the equilibrium. This is not as efficient as the Sulfur Trioxide so the reaction is much slower. The reaction mechanism in the presence of Sulfur Trioxide differs by that of excess concentrated Sulfuric Acid in that step 1 in the mechanism shown in Fig 2 below is not necessary, and the mechanism begins essentially with step 2. In both environments the SO3 is apparently the sulfonating agent (step 2 in Fig 2). This problem with the water product in this reaction can be used to our advantage if we wish to remove a sulfonyl group by using dilute Sulfuric Acid. The excess water in the diluted acid will drive this equilibrium to the left (Fig 1) according to Le Chatlier's Principle. 4- Friedel-Crafts Alkylation of Benzene and Its Derivatives Franco-American cooperation was in effect with the alkylation reaction as Professor Friedel, a French chemist, collaborated with Professor Crafts, an American chemist, to develop the Friedel Crafts Alkylation. This is an electrophilic aromatic substitution 11 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry whereby a carbocation is generated as the electrophile. There are several ways this can be done as shown in Fig 1 below. Once the carbocation has been generated then the reaction proceeds to the alkylated Benzene Ring (Fig 2) The reaction mechanism for this alkylation is shown in Fig 3 below. 12 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry There are a number of problems dealing with the alkylation reaction that lead to the development of a similar reaction, Friedel-Crafts Aycylation. The limitations to this alkylation reaction include: 1. Polysubstitution- Since the alkyl group that has been placed on the Benzene ring activates the ring toward further substitution, and each subsequent alkylation increases this activation of the ring, this leads to alkylation of the ring in several positions. This is called "polysubstitution" and leads to extremely low yields of the monosubstituted product. 2. Possible rearrangement of the initial carbocation- Carbocations will undergo molecular rearrangement where a Hydrogen will move over to an adjacent carbon with the bonding electrons (hydride shift) or a methyl group will move over to the adjacent carbon with the bonding electrons(methide shift). This molecular rearrangement within a carbocation will only occur if it results in a more stable carbocation. We know that the relative stability of carbocations runs: C6H5-CH2+ = CH3-CH=CH2+ > tertiary > secondary > primary > CH3+ 13 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry So if a hydride or methide shift results in a primary carbocation becoming a tertiary or secondary carbocation then the molecular rearrangement will occur at least partially resulting in possible multiple products for alkylation. Molecular rearrangement will not occur if it would result in a tertiary carbocation becoming a secondary or primary carbocation. 3. Benzene derivatives where the substituents deactivate the ring give very poor yields in all Friedel-Crafts reactions 4. Halobenzenes or vinylic halides cannot be used as the alkylating agent because they do not form carbocations readily. This is true for both Friedel-Crafts reactions. 5- Friedel-Crafts Acylation of Benzene and Its Derivatives An acyl group is an alkyl group attached to a carbonyl carbon: R-C=O Acylation involves the generation of the acyl group as an electrophile and substituting it upon a Benzene ring.(Fig 1) 14 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry Basic reactions Aromatic nitrations to form nitro compounds take place by generating a nitronium ion from nitric acid and sulfuric acid. Aromatic sulfonation of benzene with fuming sulfuric acid gives benzenesulfonic acid. Aromatic halogenation of benzene with bromine, chlorine or iodine gives the corresponding aryl halogen compounds catalyzed by iron tribromide. 15 Assistant Lecture Tamara Ala'a College of Dentistry Organic Chemistry The Friedel-Crafts reaction exists as an acylation and an alkylation with as reactants acyl halides or alkyl halides. The catalyst is always aluminum chloride. For example : 16