The Cardiovascular System: The Heart
advertisement

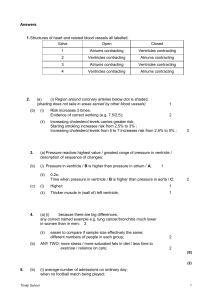
The Cardiovascular System: The Heart Function: The heart pumps blood through blood vessels to all body tissues. Anatomy of the Heart Location and Shape of the Heart The heart is in midline of the thoracic cavity. It lies in the mediastinum. The heart is cone-shaped. The apex is directed anteriorly, inferiorly and to the left. The base is directed posterior, superiorly and to the right. Pericardium The membrane that surrounds and protects the heart is pericardium. It confines the heart to its position in the mediastinum, allowing sufficient freedom of movement for contraction. Fibrous pericardium The superficial fibrous pericardium is composed of dense irregular arranged collagenous connective tissue. The fibrous pericardium prevents overstretching of the heart, provides protection and anchors the heart in the mediastinum. Serous pericardium The deeper serous pericardium is a thinner, more delicate membrane that forms a double layer around the heart. The outer parietal layer of the serous pericardium is fused to the fibrous pericardium. The inner visceral layer of serous pericardium, the epicardium, is one of the layers of the heart wall and adheres tightly to the surface of the heart. Between the parietal and visceral layers of the serous pericardium is a thin film of serous fluid, the pericardial fluid. Pericardial fluid reduces friction between the layers of the serous pericardium as the heart moves. The space that contains pericardial fluid is the pericardial cavity. Layers of the Heart Wall The wall of the heart consists of three layers; epicardium (external layer), myocardium (middle layer), and the endocardium (inner layer). The epicardium, outer layer of the heart wall, is the visceral layer of the serous pericardium. The middle myocardium, which is cardiac muscle tissue, makes up the bulk of the heart and is responsible for its pumping action. The cardiac muscle is involuntary. The cardiac muscle cells or fibers swirl diagonally around the heart in bundles. The endocardium is a thin layer of endothelium overlying a thin layer of connective tissue. The endocardium is continuous with the endothelial lining of the blood vessels. Chambers of the Heart The heart has four chambers; two superior chambers are the atria, and two inferior chambers are the ventricles. On the surface of the heart are a series of grooves, called sulci that contain coronary blood vessels. Each sulcus marks the external boundary between two chambers of the heart. The deep coronary 1 sulcus marks the boundary between the superior atria and inferior ventricles. Interventricular sulcus, is a shallow groove that marks the boundary between the right and left ventricles. Right Atrium The right atrium receives blood from three veins; the superior vena cava, inferior vena cava, and coronary sinus. Between the right atrium and left atrium is a thin partition called the interatrial septum. An oval depression called the fossa ovalis, in the interatrial septum is the remnant foramen ovale, an opening of the fetal heart that normally closes soon after birth. Blood passes from the right atrium into the right ventricle through the tricuspid or right atrioventricular (AV) valve. This AV valve consists of three cusps. Right Ventricle The inside of the right ventricle contains a series of ridges formed by raised bundles of cardiac muscle fibers called trabeculae carneae. The cusps of the tricuspid valve are connected to tendon-like cords, the chordae tendineae which in turn are connected to cone-shaped trabeculae carneae called papillary muscles. The right ventricle is separated from the left ventricle by a partition, the interventricular septum. Blood passes from the right ventricle through the pulmonary semilunar valve into the pulmonary trunk, which divides into right and left pulmonary arteries going to the lungs. Left Atrium The left atrium receives blood from the lungs through four pulmonary veins (two from each lung) coming from the lungs. Blood passes from the left atrium into the left ventricle through the bicuspid (mitral) or left atrioventricular valve which has two cusps. Left Ventricle The left ventricle forms the apex of the heart. The left ventricle contains trabeculae carneae and has chordae tendinae that anchor the cusps of the bicuspid valve to papillary muscles. Blood passes from the left ventricle through the aortic semilunar valve into the ascending aorta. The first branches off the ascending aorta are the coronary arteries which carry blood to the heart wall. The remainder of the blood passes into the arch of the aorta and the descending aorta (thoracic aorta and abdominal aorta). Myocardial Thickness and Function The thickness of the myocardium of the four chambers varies according to each chamber’s function. The thin-walled atria deliver blood into the adjacent ventricles. Ventricles pump blood greater distances, their walls are thicker. The right side pumps blood a short distance to the lungs at lower pressure, resistance to blood flow is small. The left ventricle pumps blood great distances at higher pressure, resistance to blood flow is greater. The left ventricle works much harder than the right ventricle to maintain the same rate of blood flow. The muscular wall of the left ventricle is considerably thicker than the wall of the right ventricle. Heart Valves and Circulation of Blood As each chamber of the heart contracts, it pushes a volume of blood into the ventricles or out of the ventricles into the arteries. Valves open and close in response to pressure changes as the heart contracts and relaxes. The four valves helps ensure the one-way flow of blood by opening to let blood through and then closing to prevent its backflow. 2 Operation of the Atrioventricular Valves The atrioventricular (AV) valves are located between the atria and ventricles tricuspid valve right, bicuspid (mitral) valve left.. When AV valves are open the ventricles are relaxed, blood moves through the open AV valves from a higher pressure in the atria to a lower pressure in the ventricles. When the ventricles contract the pressure of the blood drives the cusps of the AV valves upward until their edges meet and close the opening. The papillary muscles contract and pull on and tighten the chordae tendineae. This prevents the valve cusps from everting (opening into the atria) in response to high ventricular pressure. Operation of the Semilunar Valves The semilunar valves (SL), aortic and pulmonary valves are made up of three crescent shaped cusps. Each cusp attaches to the arterial wall. The SL valves allow ejection of blood from the ventricles into arteries but prevent backflow of blood into the ventricles. Pressure builds up within the chambers due to the ventricles contracting. The semilunar valves open when pressure in the ventricles exceeds the pressure in the arteries, permitting ejection of blood from the ventricles into the pulmonary trunk and aorta. The ventricles relax, blood starts to flow back toward the heart. This back flowing blood fills the valve cusps, which causes the semilunar valves to close tightly. No valves guard the junctures between the venae cavae and the right atrium or the pulmonary veins and the left atrium. Systemic and Pulmonary Circulations In postnatal (after birth) circulation, the heart pumps blood into two closed circuits – the systemic circulation and the pulmonary circulation. The two circuits are arranged in series: The output of one becomes the input of the other. The left side of the heart is the pump for the systemic circulation; it receives oxygen-rich blood from the lungs. The left ventricle ejects blood into the aorta. From the aorta, the blood enters the systemic arteries. In the tissues, the arteries give rise to smaller-diameter arterioles which lead into beds of systemic capillaries. Exchange of nutrients and gases occurs across the thin capillary walls. Blood flows through the capillaries and enters the systemic venules. The venules carry deoxygenated blood away from tissues and merge to form larger systemic veins. Ultimately the blood flows back to the right atrium. The right side of the heart is the pump for the pulmonary circulation. It receives deoxygenated blood returning from the systemic circulation. Blood ejected from the right ventricle flows into the pulmonary trunk, which branches into the right and left pulmonary arteries that carry blood to the right and left lungs. In the pulmonary capillaries, blood unloads CO2 which is exhaled and picks up inhaled O2.. Freshly oxygenated blood then flows into pulmonary veins and returns to the left atrium. Coronary Circulation The myocardium has its own network of blood vessels, the coronary or cardiac circulation. The coronary arteries branch from the ascending aorta and encircle the heart. While the heart is contracting, little blood 3 flows in the coronary arteries because they are squeezed shut. When the heart relaxes pressure of blood in the aorta propels blood through the coronary arteries into capillaries, and then into coronary veins. Coronary Arteries Two coronary arteries, right and left, branch from the ascending aorta and supply oxygenated blood to the myocardium. Where two or more arteries supply the same region, they usually connect. These connections, called anastomoses, to provide alternate routes for blood to reach a particular organ or tissue. Myocardium contains many anastomoses that connect branches of a given coronary artery or extend between branches of different coronary arteries. They provide detours for arterial blood if a main route becomes obstructed. Heart muscle then may receive sufficient oxygen and nutrients if coronary arteries are partially blocked. Coronary Veins After blood passes through the arteries of the coronary circulation, it flows into capillaries then moves into coronary veins. Deoxygenated blood from the myocardium drains into a large vascular sinus in the coronary sulcus on the posterior surface of the heart called the coronary sinus. Blood in the coronary sinus then empties into the right atrium. Myocardial Ischemia and Infarction Partial obstruction of blood flow in the coronary arteries may cause myocardial ischemia, a condition of reduced blood flow to the myocardium. Ischemia causes hypoxia (reduced O2 supply), which may weaken cells without killing them. A complete obstruction to blood flow in a coronary artery may result in a myocardial infarction, (MI), called a heart attack. Infarction means the death of an area of tissue because of interrupted blood supply. The heart muscle loses some of its strength, because the heart tissue distal to the obstruction dies and is replaced by noncontractile scar tissue. Coronary thrombosis is a clot in an unbroken vessel caused by adhesion of platelets to the roughened surface of the endothelial cells of small coronary blood vessels. This causes an intravascular clot in the vessel to block blood flow. Histology of Cardiac Muscle Tissue Review histology from A-P I. The ends of cardiac muscle fibers connect to neighboring fibers by transverse thickenings of the sarcolemma called intercalated discs. The discs contain desmosomes, which hold the fibers together and have gap junctions, which allow muscle action potentials to be conducted from one muscle fiber to the next. Autorhythmic Fibers: The Conduction System The heart has an inherent and rhythmical electrical activity. The source of this electrical activity is a network of specialized cardiac muscle fibers called autorhythmic fibers, because they are self-excitable. Autorhythmic fibers repeatedly generate action potentials that trigger heart contractions. During embryonic development 1% of the cardiac muscle fibers become autorhythmic fibers. These cardiac muscle fibers have two functions: 1. They act as a pacemaker, setting the rhythm of electrical excitation that causes contraction of the heart. 4 2. They form the conduction system, a network of specialized cardiac muscle fibers that provide a path for cardiac excitation to progress through the heart. Conduction system ensures that cardiac chambers become stimulated to contract in a coordinated forceful manner, which makes the heart an effective pump. Cardiac action potentials propagate through the conduction system in the following sequence: 1. Cardiac excitation normally begins in the sinoatrial (SA) node located in the right atrial wall. The SA node cells do not have a stable resting potential. They repeatedly depolarize to threshold spontaneously. The spontaneous depolarization is the pacemaker potential. When the pacemaker potential reaches threshold, it triggers an action potential. Each action potential from the SA node propagates throughout both atria via gap junctions in the intercalated discs of atrial muscle fibers. The atria contract following the action potential. 2. By conducting along atrial muscle fibers, the action potential reaches the atrioventricular (AV) node, located in the septum between the two atria. 3. From the AV node, the action potential enters the atrioventricular (AV) bundle (also known as the bundle of His). This bundle is the only site where action potentials can be conducted from the atria to the ventricles. 4. After propagating along the AV bundle, the action potential enters both the right and left bundle branches. The bundle branches extend through the interventricular septum toward the apex of the heart. 5. Finally, Purkinje fibers rapidly conduct the action potential from the apex of the heart upward to the remainder of the ventricular myocardium. Then the ventricles contract, pushing the blood upward toward the semilunar valves. On their own, autorhythmic fibers in the SA node would initiate action potentials about 100 times per minute. Because action potentials from the SA node spread through the conduction system and stimulate other areas before these areas are able to generate an action potential, the SA node acts as the normal pacemaker of the heart. Nerve impulses from the SNS and hormones (such as epinephrine) modify by increasing the timing and strength of each heartbeat, but they do not establish the fundamental rhythm. The PNS releasing acetylcholine slows SA node pacing to about 75 action potentials per minute. Action Potential and Contraction of Contractile Fibers The action potential initiated by the SA node travels along the conduction system and spreads out to excite atrial and ventricular contractile muscle fibers. An action potential occurs in a contractile fiber as follows: 1. Depolarization. Contractile fibers have a stable resting membrane potential that is close to -90 mV. When a contractile fiber is brought to threshold by an action potential its Na+ channels open. Opening of these channels allows Na+ ions to flow into the contractile cardiac fibers, because the cytosol of these fibers are electrically more negative than the interstitial fluid and Na+ ion concentration is higher in interstitial fluid. Inflow of Na+ ions down the electrochemical gradient produces a rapid 5 depolarization. Within a few milliseconds, the Na+ channels automatically inactivate (close) and Na+ ion inflow decreases. 2. Plateau. The next phase of an action potential in a contractile cardiac muscle fiber is the plateau, a period of maintained depolarization. It is due to opening Ca2+ channels in the sarcolemma. When these channels open, Ca2+ ions move from the interstitial fluid (which has a higher Ca2+ ion concentration) into the cytosol. This inflow of Ca2+ ions causes even more Ca2+ ions to pour out of the sarcoplasmic reticulum into the cytosol through additional Ca2+ channels in the sarcoplasmic reticulum membrane. The increased Ca2+ ion concentration in the cytosol triggers the contraction. K+ channels are also found in the sarcolemma of a contractile fiber. Just before the plateau phase begins, some of these K+ channels open, allowing K+ ions to leave the contractile fiber. Depolarization is sustained during the plateau phase because Ca2+ ion inflow just balances K+ ion outflow. The plateau phase lasts for about 0.25 sec, and the membrane potential of the contractile fiber is close to 0 mV. Depolarization in a skeletal muscle fiber is much briefer, about 1 msec because it lacks a plateau phase. 3. Repolarization. The recovery of the resting membrane potential during the repolarization phase of a cardiac action potential resembles that in other excitable cells. After a delay all of the K+ channels open. Outflow of K+ ions restores the negative resting membrane potential (-90 mV). At the same time the Ca2+ channels in the sarcolemma and the sarcoplasmic reticulum are closing, which also contributes to repolarization. The mechanism of contraction is similar in cardiac and skeletal muscle: action potential leads to contraction after a short delay. Substances that alter the movement of Ca2+ ions through Ca2+ channels influence the strength of heart contractions. Epinephrine increases contraction force by enhancing Ca2+ ion flow into the cytosol. In muscle, the refractory period is the time interval during which a second contraction cannot be triggered. The refractory period of a cardiac muscle fiber lasts longer than the contraction itself. Another contraction cannot begin until relaxation. Tetanus (maintained contraction) cannot occur in cardiac muscle as it can in skeletal muscle, if you consider how the ventricles work. Their pumping function depends on alternating contraction (when they eject blood) and relaxation (when they refill). If heart muscle could undergo tetanus, blood flow would cease. ATP Production in Cardiac Muscle Cardiac muscle produces small amount of ATP by anaerobic cellular respiration. Instead, it relies almost exclusively on aerobic cellular respiration in its numerous mitochondria. Cardiac muscle fibers use several fuels to power mitochondrial ATP production. At rest, ATP comes mainly from oxidation of fatty acids and glucose. During exercise, the heart’s use of lactic acid produced by contracting skeletal muscles increases. Cardiac muscle also produces some ATP from creatine phosphate. 6 Electrocardiogram As action potentials propagate through the heart, they generate electrical currents that can be detected at the surface of the body. Electrocardiogram (ECG or EKG) is a recording of these electrical signals. The ECG is a composite record of action potentials produced by all the cardiac muscle fibers during each heartbeat. Instrument used to record the changes is an electrocardiograph. In a typical ECG recording, three clearly recognizable waves appear with each heartbeat. The first wave, the P wave, is a small upward deflection on the ECG. The P wave represents atrial depolarization, which spreads from the SA node through contractile fibers in both atria. The P-Q segment represents steady depolarization of the atria as the atria contract. The second wave, the QRS complex, begins as a downward deflection (Q) continues as a large, upright, triangular wave (R) and ends as a downward wave (S).The QRS complex represents rapid ventricular depolarization, as the action potential spreads through ventricular contractile fibers. The S-T segment represents steady depolarization of the ventricles, as the ventricles contract. During the S-T segment the ECG tracing is flat. The third wave, the T wave, is a dome-shaped upward deflection; it represents ventricular repolarization just as the ventricles are starting to relax. The T wave is smaller than the QRS complex because repolarization occurs more slowly than depolarization. The time between waves are called intervals or segments. The P-Q or P-R interval is the time from the beginning of the P wave to the beginning of the QRS complex. It represents the time required for the action potential to travel through the atria, atrioventricular node and remaining cardiac conducting muscle fibers of the conduction system. The S-T segment represent the time when ventricular contractile fibers are depolarized during the plateau phase of the action potential. Correlation of ECG Waves with Atrial and Ventricular Systole Systole refers to the phase of contraction and diastole refers to the phase of relaxation of the heart during the cardiac cycle. The ECG waves predict the timing of atrial and ventricular systole and diastole. 1. A cardiac action potential arises in the SA node. It propagates throughout the atrial muscle and down to the AV node in about 0.08 sec. As the atrial contractile fibers depolarize, the P wave appears in the ECG. 2. After the P wave begins, the atria contract (atrial systole). Conduction of the action potential slows at the AV node. The 0.1 sec delay gives the atria time to contract, adding to the volume of blood in the ventricles before ventricular systole begins. 3. About 0.2 sec after onset of the P wave, depolarization progresses producing the QRS complex. At the same time, atrial repolarization is occurring, but it is not usually evident in an ECG because the larger QRS complex masks it. 4. Contraction of ventricular contractile fibers (ventricular systole) begins shortly after the QRS complex appears and continues during the S-T segment. As contraction proceeds from the apex toward the base of the heart, blood is squeezed upward toward the semilunar valves. 5. Repolarization of ventricular contractile fibers begins at the apex and spreads throughout the ventricular myocardium. This produces the T wave in the ECG about 0.4 sec after the onset of the P wave. 7 6. Shortly after the T wave begins, the ventricles start to relax (ventricular diastole). By 0.6 sec, ventricular repolarization is complete and ventricular contractile fibers are relaxed. During next 0.2 sec, contractile fibers in both the atria and ventricles are relaxed. At 0.8 sec, the P wave appears again in the ECG, the atria begin to contract and the cycle repeats. The Cardiac Cycle A single cardiac cycle includes all the events associated with one heartbeat. A cardiac cycle consists of systole and diastole of the atria plus systole and diastole of the ventricles. Pressure and Volume Changes During the Cardiac Cycle In each cardiac cycle, the atria and ventricles alternately contract and relax, forcing blood from areas of higher pressure to areas of lower pressure. As a chamber of the heart contracts, blood pressure within it increases. When heart rate is 75 beats/min, a cardiac cycle lasts 0.8 sec. Atrial Systole During atrial systole, about 0.1 sec, the atria are contracting. At the same time, the ventricles are relaxed in ventricular diastole. 1. Depolarization of the SA node causes atrial depolarization, marked by the P wave in the ECG. 2. Atrial depolarization causes atrial systole. As the atria contract, pressure on the blood within the atria forces blood through the open AV valves into the ventricles. 3. Atrial systole contributes a final 25 mL of blood to the volume already in each ventricle (about 105 mL). The end of atrial systole is also the end of ventricular diastole. Each ventricle contains about 130 mL at the end of its relaxation period (diastole). This blood volume is end-diastolic volume (EDV). 4. The QRS complex in the ECG marks the onset of ventricular depolarization. Ventricular Systole During ventricular systole, about 0.3 sec, the ventricles are contracting. At the same time the atria are relaxed in atrial diastole. 5. Ventricular depolarization causes ventricular systole. As ventricular systole begins, pressure rises inside the ventricles and pushes blood up against the atrioventricular (AV) valves, forcing these valves shut. For about 0.05 seconds, SL and AV valves are closed. This is the period of isovolumetric contraction. The cardiac muscle fibers are contracting and exerting force but are not yet shortening. The muscle contraction is isometric. All four valves are closed and the ventricular volume remains the same (isovolumetric). 6. Continued contraction of the ventricles causes pressure inside the chambers to rise. When left ventricular pressure surpasses aortic pressure and right ventricular pressure rises above the pressure in the pulmonary trunk, SL valves open. At this point, ejection of blood from the heart begins. The period when the SL valves are open is ventricular ejection and lasts for about 0.25 sec. The pressure in the left ventricle continues to rise to about 120mm Hg. 8 7. The left ventricle ejects about 70mL of blood into the aorta and the right ventricle ejects the same volume of blood into the pulmonary trunk. The volume remaining in each ventricle at the end of systole, about 60mL, is the end-systolic volume (ESV). Stroke volume (SV), the volume ejected per beat from each ventricle, equals end-diastolic volume minus end-systolic volume; SV = EDV – ESV. At rest, the SV is about 70 mL, 130 mL – 60 mL. 8. T wave in the ECG marks the onset of ventricular repolarization. Relaxation Period During the relaxation period, about 0.4 sec, atria and the ventricles are both relaxed. 9. Ventricular repolarization causes ventricular diastole. As the ventricles relax, pressure within the chambers falls, and blood in the aorta and pulmonary trunk begins to flow backward toward the regions of lower pressure in the ventricles. Back flowing blood catches in the valve cusps and closes the SL valves. Rebound of blood off the closed cusps of the aortic valve produces the dicrotic notch or wave on the aortic pressure curve. After the SL valves close, ventricular volume does not change because all four valves are closed. This is a period of isovolumetric relaxation. 10. As the ventricles continue to relax, pressure falls. When ventricular pressure drops below atrial pressure AV valves open, and ventricular filling begins. The major part of ventricular filling occurs just after the AV valves open. Blood that has been flowing into and building up in the atria during atrial diastole now rushes rapidly into the ventricles. At the end of the relaxation period ventricles are about ¾ full. The P wave appears in the ECG, the start of another cardiac cycle. Heart Sounds Auscultation the act of listening to sounds within the body is done with a stethoscope. Sound of the heartbeat comes primarily from blood turbulence caused by the closing of the heart valves. During each cardiac cycle, there are four heart sounds, in a normal heart only the first and second heart sounds (S1 and S2) are loud enough to be heard. The first sound (S1) a lubb sound, is louder and a bit longer than the second sound. S1 is caused by blood turbulence associated with closure of the AV valves soon after ventricular systole begins. The second sound (S2) is shorter than and not as loud as the first, a dupp sound. S2 is caused by blood turbulence associated with closure of the SL valves at the beginning of ventricular diastole. S3 is due to blood turbulence during rapid ventricular filling, and S4 is due to blood turbulence during atrial systole. Cardiac Output Although the heart has autorhythmic fibers that enable it to beat independently, its operation is governed by events occurring throughout the body. The workload of the heart varies. 9 Cardiac output (CO) is the volume of blood ejected from the left ventricle into the aorta each minute. Cardiac output equals the stroke volume (SV), the volume of blood ejected by the ventricle during each contraction, multiplied by the heart rate (HR), the number of heartbeats per minute: SV = EDV – ESV 70mL = 130mL – 60mL CO (mL/min) = SV x (mL/beat) HR (beats/min) In a resting adult male, SV averages 70 mL/beat, and heat rate is about 75 beats/min. Average cardiac output is 5.25 L/min. CO = 70 mL/beat x 75 beats/min = 5250 mL/ min or 5.25 L/min This volume is close to total blood volume of 5 liters in an adult. Thus your entire blood volume flows through your pulmonary and systemic circulations each minute. There are factors that increase stroke volume and/or heart rate so as to normally increase CO. Cardiac reserve is the difference between a person’s maximum cardiac output and cardiac output at rest. Average person has a cardiac reserve of four or five times the resting value. Regulation of Stroke Volume A healthy heart will pump out the blood that entered its chambers from the previous diastole. If more blood returns to the ventricles during diastole, then more blood is ejected during the next systole. Three factors regulate stroke volume and ensure that the left and right ventricles pump equal volumes of blood: 1. Preload, the degree of stretch on the heart before it contracts; 2. Contractility, forcefulness of contraction of individual ventricular muscle fibers; 3. Afterload, the pressure that must be developed by the ventricles before ejection of blood from the ventricles can occur. Preload: Effect of Stretching A greater preload (stretch) on cardiac muscle fibers prior to contraction increases their force of contraction. The more the ventricles fill with blood during diastole, the greater the force of contraction during systole. This relationship is Frank-Starling law of the heart. Preload is proportional to the enddiastolic volume (EDV) (the volume of blood that fills the ventricles at the end of diastole). The greater the EDV the more forceful the next contraction. Two factors determine EDV: 1.The duration of ventricular diastole. When heart rate increases, duration of diastole is shorter. Less filling time means a smaller EDV and the ventricles may contract before they are adequately filled. 2. Systemic venous return, the volume of blood returning to the right ventricle. When venous return increases, a greater volume of blood flows into the ventricles, and the EDV is increased. The Frank-Starling law of the heart explains the equal output of the right and left ventricles and the same volume of blood flowing to both the systemic and pulmonary circulations. If the left side of the heart pumps a little more blood than the right side, the volume of blood returning to the right ventricle (venous 10 return) increases. Increased EDV causes the right ventricle to contract more forcefully on the next beat, bringing the two sides back into balance. Contractility The second factor that influences stroke volume is myocardial contractility, the strength of contraction at any given preload or stretch. Substances that increase contractility are positive inotropic agents (prefix ino-muscle fiber); those that decrease contractility are negative inotropic agents. Strength of contraction and stroke volume increase when a positive inotropic substance is present. Positive inotropic agents promote Ca2+ ion inflow into cardiac muscle fibers which strengthens the force of the contraction. Stimulation of the SNS which releases epinephrine and norepinephrine increased Ca2+ ion level in the interstitial fluid, and the drug digitalis have positive inotropic effects. Inhibition of the SNS, anoxia, acidosis, anesthetics, and increased K+ ion level in the interstitial fluid have negative inotropic effects. Calcium channel blockers are drugs that can have a negative inotropic effect by reducing Ca2+ ion inflow, thereby decreasing the strength of the heartbeat. Afterload Ejection of blood from the heart begins when pressure in the left ventricle exceeds the pressure in the aorta. Pressure developed by the ventricles cause blood to push the SL valves open is the afterload. An increase in afterload due to blood left in the arteries and increased pressure in the arteries causes stroke volume to decrease. Since more blood remains in the ventricles at the end of systole the cardiac muscle has to contract more forcefully to push the blood out of the ventricles into the arteries.. Conditions that can increase afterload include hypertension (elevated blood pressure) and narrowing of arteries by atherosclerosis. Regulation of Heart Rate Factors that contribute to regulation of heart rate are the autonomic nervous system innervations (SNS and PNS) and hormones released by the adrenal medulla (epinephrine and norepinephrine). Autonomic Regulation of Heart Rate Nervous system regulation of the heart originates in the cardiovascular center in the medulla oblongata. This region of the brain stem receives input from a variety of sensory receptors and from higher brain centers, limbic system (this system is concerned with various aspects of emotion and behavior) and cerebral cortex. The cardiovascular center then directs appropriate output by changing the frequency of nerve impulses in both the sympathetic and parasympathetic branches of ANS. Sensory input into CV center Even before physical activity begins, heart rate may climb. This anticipatory increase occurs because the limbic system sends nerve impulses to the cardiovascular center in the medulla. As physical activity begins, proprioceptors in the limbs and muscles send nerve impulses at an increased frequency to the cardiovascular center. Other sensory receptors include chemoreceptors, which monitor chemical changes in the blood, baroreceptors, which monitor the stretching of major arteries and veins caused by the pressure of the blood flowing through them. The baroreceptors are located in the arch of the aorta and in 11 the carotid arteries detect changes in blood pressure and provide input to the cardiovascular center when blood pressure changes. Motor output from the CV center Sympathetic neurons extend from the medulla oblongata into the spinal cord. From the thoracic region of the spinal cord, sympathetic cardiac accelerator nerves extend out to the SA node, AV node, and most portions of the myocardium. Impulses in the cardiac accelerator nerves trigger the release of norepinephrine, which binds to receptors on cardiac muscle fibers. This interaction has two separate effects: 1. In SA (and AV) node fibers, norepinephrine speeds the rate of spontaneous depolarization so that these pacemaker cardiac muscle fibers fire impulses more rapidly and heart rate increases; 2. In contractile fibers of the atria and ventricles, norepinephrine enhances Ca2+ ion entry increasing contractility. As a result, of greater strength of contraction a greater volume of blood is ejected during systole. Parasympathetic nerve impulses reach the heart via vagus (X) nerves. Vagal neurons terminate in the SA node, AV node, and atrial myocardium. These neurons release acetylcholine, which decreases heart rate by slowing the rate of spontaneous depolarization in autorhythmic fibers. Only a few vagal fibers innervate ventricular muscle, changes in parasympathetic activity have little effect on contractility of the ventricles. A balance exists between sympathetic and parasympathetic stimulation of the heart. At rest, parasympathetic stimulation predominates. Chemical Regulation of Heart Rate Chemical influence both the strength of contraction of cardiac muscle and the heart rate. Hypoxia (lowered oxygen level), acidosis (low pH) all depress cardiac activity. Several hormones and cations have major effects on the heart: 1. Hormones. Epinephrine and norepinephrine from the adrenal medulla enhance the heart’s pumping effectiveness. These hormones affect cardiac muscle fibers as they increase both heart rate and contractility. Thyroid hormones also enhance cardiac contractility and increase heart rate. . 2. Cations. Concentrations of K+, Ca2+, and Na+ ions have an effect on cardiac function. Elevated blood levels of K+ or Na+ ions decrease heart rate and contractility. Excess Na+ ions blocks Ca2+ ion inflow during cardiac action potential propagation decreasing the force of contraction. Excess K+ ions block generation of action potentials. A moderate increase in interstitial Ca2+ ion level increases heart rate and strengthens the heartbeat. Other Factors in Heart Rate Regulation Age, gender, physical fitness, and body temperature also influence heart rate. Increased body temperature causes the SA node to discharge impulses more quickly, increasing heart rate. Decreased body temperature decreases heart rate and strength. Arrhythmias: Disorders of Cardiac Rhythms Tachycardia is an elevated heart rate – a resting HR > 100beats/min. Bradycardia is a slow heart rate – a resting HR < 50-60beats/min. 12 Ventricular fibrillation – premature ventricular contractions. It is quivering of the ventricular wall and the heart stops pumping blood. The ventricular muscle cells are stimulating each other at a rapid rate. Complete (third degree) heart block – atrial and ventricular activities are no longer synchronized. Atria and ventricles beat at their own pace. Abnormal S-T segment – The S-T segment elevated above the baseline in myocardial infarction (an area damaged cardiac tissue) and depressed below the baseline in myocardial hypoxia. Exercise and the Heart An untrained person increases CO by increasing SV, HR and BP. A well-trained athlete has a CO double that of a sedentary person. Training causes hypertrophy (enlargement) of the heart. In this person, stroke volume is increased while heart rate is decreased. A trained person has increased CO because of increased SV decreased HR and BP. 13