Elements of Chemistry: Carbon the Element of Life
advertisement

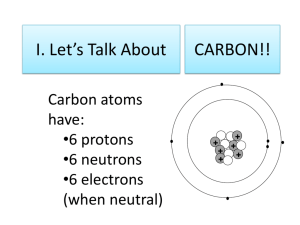
Elements of Chemistry: Carbon the Element of Life : 58 What is organic chemistry? 1:05 What makes carbon so important? 1:28 What percent of all substances contain carbon? 2:29 How many electrons does carbon have? How many valence electrons does carbon have? 2:45 What is the octet rule? 2:58 How does carbon satisfy the octet rule? 3:05 Name the bonding options for carbon. 1. 2. 3. 3:28 What adjectives describe the bonds that carbon can form? 3:39 Name 3 elements other than carbon itself that carbon commonly bonds with. 1. 2. 3. 4:02 Hydrocarbons 4:16 What are hydrocarbons? 4:26 Where do most deposits of hydrocarbons exist in the Earth? 4:36 Name 3 purposes of hydrocarbons. 1. 2. 3. 4:42 Natural gas and petroleum are produces by what? 4:50 What name is used to refer to these hydrocarbons? 4:59-5:16 What is the simplest hydrocarbon? Draw a model. 5:48 Methane, ethane and propane are part of what family of hydrocarbons? 5:56 What 2 things do methane, ethane, propane, hexane and pentane have in common? 1. 2. 6:19 Draw a branched alkane. 6:41-7:00 What is the general formula for alkanes? What does n mean? 7:04 If we have 4 carbon atoms, how many hydrogens are there? 7:17 Cycloalkanes contain what shape? 7:25 What is the general formula for cycloalkanes? 7:31 What are saturated hydrocarbons? 7:37 What are unsaturated hydrocarbons? 7:51 Draw ethane. What is the formula? 8:00-8:10 What is an alkene? Formula? 8:21-8:31 What is an alkyne? Formula? 8:47 Fill in the table that explains how the name indicates the number of carbon atoms. 8:52 Fill in the table that explains how the name indicates the kind of covalent bond. 9:32 Functional Group 10:06 What is a halocarbon? 10:27 Draw CHCl3 (trichloromethane) 10:42 What do all these parts of the word mean? Tri chloro meth ane 11:09 What is the hydroxyl group? 11:14 Compounds that contain the hydroxyl group are called? 11:36 What do all these parts of the word mean? meth an 11:54 What kind of alcohol is in beer and wine and some vehicle fuels? 12:08 What is ether? 12:23 What is the carbonyl group? 12:57 What two classes of molecules are bonded with nitrogen? 1. 2. 13:09 Polymers and Plastics ol 13:09-13:38 What do we call molecules with a backbone of carbon? 13:38-13:42 What makes up polymers? Which kind makes up cellulose? 14:05 What do we call synthetic polymers? 14:10 What was the first synthetic polymers? 14:16 Name two developed in the 1930s 1. 2. 14:56 What are the benefits of synthetic polymers? 15:08 What are the disadvantages of synthetic polymers? 15:27 Life Processes 15:52 List the chemical processes of life 16:15 What is the most fundamental life process? 16:25 What is the ultimate source of energy? 16:35 What plants organelle is responsible for photosynthesis? 16:42 Write the chemical equation for photosynthesis. 16:51 What is glucose used for in living systems? 16:54 What is the byproduct of photosynthesis? 17:31 How does a carnivore obtain energy if they do not eat plants? 17:54 Quiz 1. 2. 3. 4. 5. 6. 7. 8. 9. 10.