Molecular Orbital Analysis of Diels-Alder reaction
advertisement
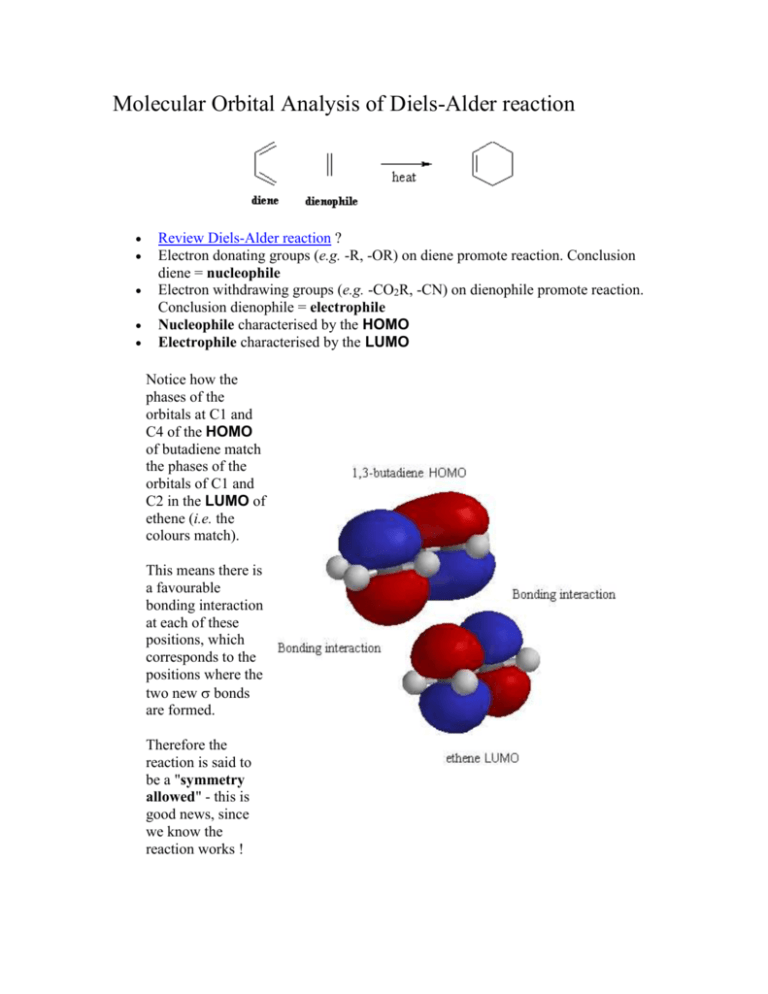
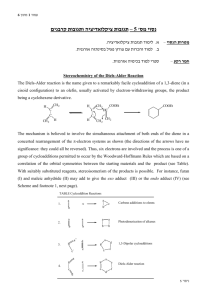
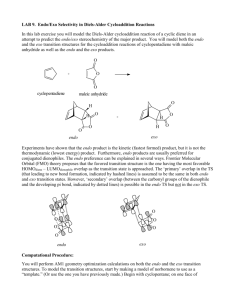
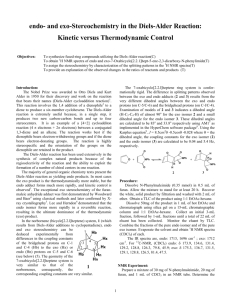
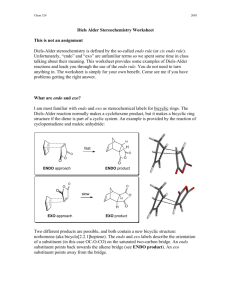
Molecular Orbital Analysis of Diels-Alder reaction Review Diels-Alder reaction ? Electron donating groups (e.g. -R, -OR) on diene promote reaction. Conclusion diene = nucleophile Electron withdrawing groups (e.g. -CO2R, -CN) on dienophile promote reaction. Conclusion dienophile = electrophile Nucleophile characterised by the HOMO Electrophile characterised by the LUMO Notice how the phases of the orbitals at C1 and C4 of the HOMO of butadiene match the phases of the orbitals of C1 and C2 in the LUMO of ethene (i.e. the colours match). This means there is a favourable bonding interaction at each of these positions, which corresponds to the positions where the two new bonds are formed. Therefore the reaction is said to be a "symmetry allowed" - this is good news, since we know the reaction works ! Frontier - Molecular Orbitals A useful molecular orbital model for analyzing pericyclic reactions has been proposed by Kenichi Fukui of Japan. This frontier-orbital approach is based on the assumption that bonds are formed by a flow of electrons from the highest occupied molecular orbital (HOMO) of one reactant or participating bond to the lowest unoccupied molecular orbital (LUMO) of another reactant or bond. To illustrate, consider the [4+2] cycloaddition of 1,3-butadiene and ethylene.to give cyclohexene. The pertinent molecular orbitals involved in this reaction were described elsewhere, and the two combinations of HOMO and LUMO are shown in the following diagram. Note that regardless of which combination is examined, the terminal orbital phases match, indicating a bonding interaction. Since the dienophile often has electron-withdrawing substituents and the diene is usually electron rich, the electron flow pattern on the left seems to best represent the course of most Diel-Alder reactions. This frontier orbital approach to cycloaddition reactions is general, and is simple to apply thanks to the alternation of terminal orbital phase relationships as a polyene changes from a 4n electron system to a 4n + 2 electron system. By clicking on the above diagram, these phase relationships will be displayed for HOMO and LUMO of polyenes in both classes. Only the terminal orbital phases (colored in the diagram) are important for frontier orbital analysis. The frontier orbital analysis of a [6s + 2s] cycloaddition reaction will be demonstrated by clicking on the diagram a second time. An antibonding node is present in both HOMO-LUMO combinations (one is shown), so this reaction is orbital symmetry forbidden. An additional feature of this treatment of cycloaddition reactions is its rationalization of the tendency of Diels-Alder reactions of cyclic dienes to form endo adducts preferentially. This was noted earlier, and is further illustrated in the following diagram. The first two equations are straightforward examples of the endo predilection of substituents or rings (colored green) attached to a bicyclic ring system. The third equation shows a more subtile case of the same orientational factor, which essentially favors that [4+2] transition state in which unsaturated substituents on the dienophile are directed toward the diene double bonds. By clicking on this diagram, the secondary orbital bonding interaction that stabilizes the endo transition state for a typical DielsAlder reaction will be displayed. Not all cycloaddition reactions favor endo products. The predominant product from the [6s + 4s] reaction shown earlier is the exo adduct. Frontier orbital analysis of this case demonstrates that secondary orbital interaction destabilizes the endo transition state. Endo - Exo Selectivity When both the diene and the dienophile are suitably substituted, a stereochemical feature arises because the reactants may approach each other in two distinct orientations. The substituent on the dienophile may be directed away from the diene (exo approach) or toward the diene (endo approach). This stereochemical difference is often found with cyclic compounds. One very simple exemple is the Diels-Alder addition of two cyclopentadiene(1) (Figure 3). Figure 3 : Diels-Alder addition of two cyclopentadienes 1. Mechanisms In most Diels-Alder reactions, when the product distribution is under kinetic control, the endo adduct is preferentially, sometimes exclusively, formed. Several hypotheses have been made to explain this selectivity : o o o Alder[5] proposed that endo addition was the consequence of a plane-toplane orientation of diene and dienophile with "maximum accumulation of double bonds". Since this same orientation would promote stability in a molecular complex, it has been suggested that complex formation between the reactants may be responsible for preferential endo addition. Woodward and Hoffmann[6] ascribe endo addition to interaction of occupied orbitals with unoccupied orbitals, the endo transition state conformation being favored by orbital symmetry relative to the exo conformation. With the model of frontier orbitals, we have interaction of HOMOs (Highest Occupied Molecular Orbitals) with LUMOs (Lowest Unoccupied Molecular Orbitals). Those interactions are represented in figure 4. The main interactions that will form the bonds are shown by plain bold lines, the secondary interactions, responsible for the endo-exo selectivity, are shown by small squiggly lines. We do not know if the molecules approch so closely that the secondary interactions can be as important as shown in figure 1. Studies of Diels-Alder transition states can also give an explanation of this selectivity[7]. The transition state in an asynchronous mechanism can be described using several resonance structures (figure 5). One of this resonance structure is a zwitterion. In the endo transition state, the proximity of the two charges stabilizes this structure and thus favours the endo addition. For the addition of the two cyclopentadienes, the forming bond lengths are 2.183 Å and 2.119 Å in the exo transition state and 2.184 Å and 2.127 Å in the endo transition state. This prove that the mechanism for this addition is asynchronous, and that the endo transition state is more assymetric, ie. the zwitterion resonance strucure is more favorized than in the exo attack. Exo-Attack Endo-Attack Figure 4 : HOMO-LUMO interactions in the Diels-Alder addition of two cyclopentadienes In the above figure : o o Red lines represent single bonds Yellow lines represens double bonds o Hydrogen have been omitted o Red and yellow orbitals represent the LUMOs o Blue and green orbitals represent the HOMOs exo Transition State endo Transition State