Diels Alder Stereochemistry Worksheet This is not an assignment
advertisement

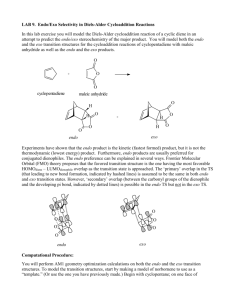
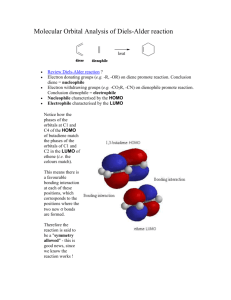
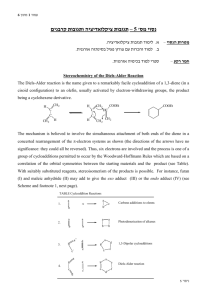
Chem 324 2005 Diels Alder Stereochemistry Worksheet This is not an assignment Diels-Alder stereochemistry is defined by the so-called endo rule (or cis endo rule). Unfortunately, “endo” and “exo” are unfamiliar terms so we spent some time in class talking about their meaning. This worksheet provides some examples of Diels-Alder reactions and leads you through the use of the endo rule. You do not need to turn anything in. The worksheet is simply for your own benefit. Come see me if you have problems getting the right answer. What are endo and exo? I am most familiar with endo and exo as stereochemical labels for bicyclic rings. The Diels-Alder reaction normally makes a cyclohexene product, but it makes a bicyclic ring structure if the diene is part of a cyclic system. An example is provided by the reaction of cyclopentadiene and maleic anhydride: H H fast O O O O O O ENDO product ENDO approach O O O O slow O H H EXO approach O EXO product Two different products are possible, and both contain a new bicyclic structure: norbornene (aka bicyclo[2.2.1]heptene). The endo and exo labels describe the orientation of a substituent (in this case OC-O-CO) on the saturated two-carbon bridge. An endo substituent points back towards the alkene bridge (see ENDO product). An exo substituent points away from the bridge. Chem 324 2005 The endo rule The endo rule for predicting Diels-Alder stereochemistry applies to reactions involving a dienophile with one or more unsaturated substituents. The rule states that the kinetic product is obtained from a transition state in which the dienophile substituent is “endo” with respect to the diene. In the case of a cyclic diene, like the one shown above, the meaning of “endo” is straightforward and so is application of the endo rule. You can just draw products with endo unsaturated substituents (that is, endo with respect to the alkene bridge). The interpretation of “endo” is far less obvious for acyclic dienes. In such cases, one needs to compare the two approaches and notice the orientation of the substituent relative to the diene. The endo approach places the substituent over (or cis to) the diene. B B A unsat A unsat unsat unsat B A ENDO approach B A EXO approach A method to this madness? The best way to use the endo rule is to be as methodical as possible. Here is one approach that will help you make accurate predictions: 1. Identify the unsaturated substituent in your dienophile 2. Draw an ENDO approach (or build a model). Make sure you line up the carbons that form the new sigma bonds (dashed lines, see above), and place the unsaturated substituent over the pi system of the diene. 3. Draw (or build) a BOAT-SHAPED ENDO product. This should be a new drawing (or model). It should use the geometry from step 2, but the bond pattern should be that of the cyclohexene product. The combination of geometry and bond pattern should give you a boat cyclohexene. 4. Re-draw (or adjust your model) so that the ring is flat (half-chair). What is the configuration of each chiral center? What is the relative stereochemistry of ring substituents? Consider the reaction between trans-1-methoxy-1,3-butadiene and acrylonitrile: Chem 324 2005 OMe OMe C N CN Step #1 identifies the unsaturated substituent as CN. Steps #2-4 are represented by the three images shown below (starting on the left, there is an ENDO approach, a BOATSHAPED ENDO product, and a flattened version of the same product): Æ Æ It is a little hard to see 3-D relationships in these images, so you should work through the problem on your own and refer to these drawings as guideposts to keep you on track. By the way, I am not advocating Spartan as the best tool for solving these problems. It was simply a convenient way for me to generate models and pictures for this worksheet. If you want to give Spartan a try, here are some tips: 9 ENDO approach. I built this model in two stages. First, I build the diene. To add a separate dienophile, I clicked alkene, the Insert button, and then I clicked inside the building area. Normal mouse operations rotate/translate the entire model. To maneuver one piece of the model, click on a piece to make it active, and press CTRL while you move the mouse. 9 BOAT-SHAPED ENDO product. There is no simple way to add bonds between the two pieces in the model. I got around this problem by searching Spartan’s library of transition states (when Spartan finds a match, it replaces the two pieces with a single model containing partial bonds). To search the library, I added curved arrows (transition button) to the ENDO approach model, and then clicked the double curved arrow button in the lower right. This gave me a transition state model with partial single and double bonds. I changed these partial bonds to the “product” bonds by clicking on Expert, selecting the desired bond type, and double-clicking the desired partial bond. 9 Flattened product. This was easy. I just clicked the minimize button. The in-class discussion identified two principal stumbling blocks: 1) visualizing the endo approach, and 2) seeing stereochemical relationships in the product. My method will help you with both, but you need to practice it and you need to be methodical. Avoid shortcuts. Chem 324 2005 Examples 1. Oakes et al., Chem. Commun., 1999, 1459. They studied Diels-Alder reactions between cyclopentadiene and acrylate esters, CH2=CHCO2R, in different media. They discovered that, using supercritical CO2 (scCO2) as the reaction medium, they could vary endo:exo from 3:1 to 4:1 by varying the external pressure. (Note, scCO2 is a “supercritical fluid” with liquid and gas-like properties; the latter includes compressibility.) They also investigated the effect of Lewis acid catalysts on endo:exo ratios. They found that a Sc+3 salt raised endo:exo to 10:1 (in toluene), 11:1 (in chloroform), and 24:1 (in scCO2). Æ Draw the endo and exo products. 2. MacMillan et al., J. Amer. Chem. Soc., 2002. They investigated cycloadditions between ethyl vinyl ketone, EtCOCH=CH2, and several simple dienes. As a rule, endo selectivity was not very high. They reasoned that making the dienophile more electronpoor should improve selectivity (and reaction rate, and yield) and this might be achieved by converting the carbonyl group, C=O, into an iminium ion, C=NR2+. They also reasoned that it should be possible to produce the iminium ion catalytically by combining the ketone with a small amount of acid and a small amount of a chiral amine, HNRR’. Their best chiral amine turned out to be: O N N H O Two “typical” experiments are described below (both gave endo:exo >200:1, %ee > 90). Æ Draw the missing products (assume “ortho/para” rule and “endo” rule both operate). O OMe .2 eq cat Et O >200:1, 96%ee .2 eq HClO4 EtOH, -30oC .2 eq cat Et .2 eq HClO4 EtOH, -30oC >200:1, 90%ee 3. Stoodley et al., Chem. Commun., 1997, 1371. They studied the reaction shown below. Endo selectivity was relatively modest (and certain ring substituents could even reverse the selectivity), but ortho/para regioselectivity was very high. Æ Which reactions give ortho products? Para products? Which product is endo and which is exo? Chem 324 2005 CO2Me MeO2C X Y MeO2C Y + O2N Y X = OMe X = OSiMe3 X = OAc X = OMe O2N Y=H Y=H Y=H Y = OSiMe3 O2N X X 67 68 > 95 35 33 32 5 65 4. Cho et al., Tet. Lett., 2001, 42, 8193. These chemists investigated inverse electron demand Diels-Alder reactions involving 3,5-dibromo-2-pyrone (“diene”) and various cyclic silyl enol ethers. The dienophiles differed only in their ring size, which ranged from 4-8 atoms. All of the reactions occurred slowly (1-3 days) and in good yields (>73%). Most dienophiles gave mainly the endo product (>90:1), but the cyclooctene gave mainly the exo product (2:1). I have drawn the endo product obtained from the cyclopentenyl silyl enol ether. Æ What is the diene’s structure? Does this reaction obey the ortho/para rule? The authors say the reaction obeys the endo rule (except for the cyclooctene reactant); how do you think they define endo? Br OSiMe3 Br OSiMe3 O + diene O 5. Gorman et al., Chem. Commun., 1998, 25. Another inverse electron demand reaction. 4:5 ratios were 99:1 for various R = Et, i-Bu, n-Bu in the presence of the iron catalyst (96 h at 23 oC, or 18 h at 60 oC), but no reaction occurred without the catalyst. Æ Do these reactions obey the ortho/para rule? The endo rule? O CO2Et OR Fe(O2CR')3 O O OR + OR + CO2Et CO2Et 4 5 Answers You can view answers to these problems by downloading the answer sheet from the 324 Molecular Models Download page. http://academic.reed.edu/chemistry/alan/324/models.html