endo and exo Stereochemistry in the Diels
advertisement

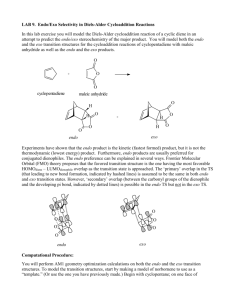
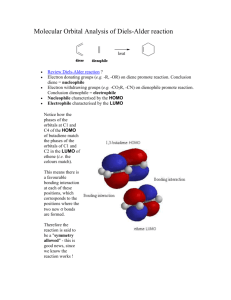
endo- and exo-Stereochemistry in the Diels-Alder Reaction: Kinetic versus Thermodynamic Control Objectives: To synthesize fused-ring compounds utilizing the Diels-Alder reaction(C) To obtain 1H NMR spectra of endo and exo-7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxy-N-phenylimide(T) To assign the stereochemistry by characterization of the splitting patterns in the 1H NMR spectra(T) To provide an explanation of the observed changes in the ratios of reactants and products (T) The 7-oxabicyclo[2.2.l]heptene ring system is conformationally rigid. The difference in splitting patterns observed between the exo and endo adducts (2 and 3) results from the very different dihedral angles between the exo and endo protons (on C-5/C-6) and the bridgehead protons (on C-l/C-4). Examination of models of 2 and 3 indicates a dihedral angle (H-C5-C4-H) of almost 90o for the exo isomer 2 and a small dihedral angle for the endo isomer 3. These dihedral angles are calculated to be 83o and 33.8o respectively using AM17 as implemented in the HyperChem software package8. Using the Karplus equation9, J = 8.5cos2θ -0.5cosθ -0.028 where θ = the dihedral angle, the coupling constants for the exo isomer (2) and the endo isomer (3) are calculated to be 0.04 and 5.4 Hz, respectively. Introduction: The Nobel Prize was awarded to Otto Diels and Kurt Alder in 1950 for their discovery and work on the reaction that bears their names (Diels-Alder cycloaddition reaction)1. This reaction involves the 1,4 addition of a dienophile2 to a diene to produce a six-member cyclohexene. The Diels-Alder reaction is extremely useful because, in a single step, it produces two new carbon-carbon bonds and up to four stereocenters. It is an example of a [4+2] cycloadditon reaction (4 π electrons + 2π electrons) between a conjugated 1,3-diene and an alkene. The reaction works best if the dienophile bears electron-withdrawing groups and if the diene bears electron-donating groups. The reaction is highly stereospecific and the orientation of the groups on the dienophile are retained in the product. The Diels-Alder reaction has been used extensively in the synthesis of complex natural products because of the regioselectivity of the reaction and the ability to exploit the formation of a number of chiral centers in one reaction. The majority of general organic chemistry texts present the Diels-Alder reaction as yielding endo products. In most cases the exo product is the thermodynamically more stable, but the endo adduct forms much more rapidly, and kinetic control is observed3. The exceptional exo stereochemistry of the furanmaleic anhydride adduct was first demonstrated by Woodward and Baer4 using classical methods and later confirmed by Xray crystallography5. Lee and Herndon6 demonstrated that the endo isomer forms more rapidly in a reversible reaction, resulting in the ultimate dominance of the thermodynamic (exo) product. In the norbornene (bicycle[2.2.l]heptene) system, 1 (which results from Diels-Alder additions to cyclopentadiene), endo and exo stereochemistry can be 7 Hb deduced experimentally from differences in the coupling constants Hx 3 4 5 of the bridgehead protons on C-1 Hx and C-4 (Hb) to the exo (Hx) or 2 1 6 endo (Hn) protons on C-5 and C-6 Hn (see below) (5). The geometry of the Hb Hn 7-oxabicyclo[2.2.l]heptene system is very similar to that of the 1 norbornenes, consequently, the corresponding coupling constants are very similar. O O + NPh NPh H O O O O H HO + O NPh H 2 O 3 Procedure: Dissolve N-Phenylmaleimide (0.35 mmol) in 0.5 mL of furan. Allow the mixture to stand for at least 20 h. Recover the white, solid product by filtration and washed with 2 mL of ether. Obtain a TLC of the product using 1:1 EtOAc-hexane. Dissolve 50mg of the product in 1 mL of hot EtOAc and chromatograph using silica gel on a 15-mL chromatographic column and 1:1 EtOAc-hexane. Collect an initial 3-mL fraction, followed by 1-mL fractions until a total of 22 mL of eluant has been collected. Monitor the eluant by TLC. Combine the fractions of the pure endo isomer and of the pure exo isomer. Evaporate the solvent and obtain 1H NMR spectra (CDCl3) of each. The IR spectra are, endo: 1713, 1696 cm-1 ; exo: 1712 -1 cm . For 13C-NMR, (CDCl3) endo: δ 173.9, 134.6, 131.4, 129.2, 128.8, 126.3, 79.8, 45.9; exo: δ 175.3, 136.7, 131.5, 129.1, 128.8, 126.5, 81.4, 47.5. NMR Experiment: Prepare a mixture of 30 mg of N-phenylmaleimide, 20 mg of furan, and 1 mL of CDCl3 in an NMR tube. Determine the 1 spectrum immediately after mixing. Store the reaction tube either at room temperature (~23oC) or at 0oC. Obtain spectra at 1 week intervals for a period of four weeks. Cleaning Up: Place the elution solvent mixture in the organic solvents container. Place the used silica gel in the solid waste container. You are responsible for completing the accompanying pre-lab exercise prior to the start of the 2nd laboratory period. 1 Diels, O., Alder, K. Liebigs Ann. Chem., 460, 98 (1928) 2 to have an affinity for dienes, from the Greek philos, meaning loving. Computational Chemistry: From the 1H NMR spectra of the isolated products, molecular models and the Karplus equation9, deduce the structures and assign the stereochemistry to each isomer. 3 Woodward, R. B., Hoffmann, R. The Conservation of Orbital Symmetry, Chemie, Weinheim, Germany, 1970. 4 Woodward, R. B., Baer, R., J. Am. Chem. Soc. 70, 1161-1166 (1948). Product Analysis: From the 1H NMR spectra of the reaction mixture, follow the reaction by obtaining the integrals of unreacted Nphenylmaleimide and of the endo and exo products. When preparing your report you should use your own data to present an analysis of the observed changes in the ratios of reactants and products. You should also consider your results relative to the average results of all people conducting the measurements at the same reaction temperature. Finally, you should comment on your results compared to the average results obtained at the other reaction temperature. Use your data and the group data to present an explanation of the observed changes in the ratios of reactants and products. Use the appended Excel spread sheet to record your data and send it as an attachment to the lead instructor for the experiment. 5 Baggio. S., Barriola, A., de Perazzo, P. K., J Chem. Soc. Perkin Trans 2, 934 (1972). 6 Lee, M. W., Herndon, W. C., J Org. Chem.,43, 518 (1978). 7 Stewart, J. J. P., J Comp. Aided Mol. Design 4, 1 (1990) 8 HyperChem for Windows, Hypercube (1994) 9 Silverstein, R. M., Bassler, G. C., Morrill, T., Spectrometric Identification of Organic Compounds, 5th edition, Wiley, New York, p. 197 (1991) 2 SECTION________________ NAME_______________________ PRE-LAB EXERCISE (due beginning of 2nd lab period) 1. Provide complete answers to the following questions in the spaces provided. i) Considering the Karplus equation and the dihedral angles, what patterns (not necessary multiplicities) would you expect for the protons on C5/C6 and on C1/C4 in a 1 H NMR spectrum of the exo and endo isomers of the Diels-Alder product? ii) Based on your expectations (above), assign the peaks observed in the region of the 1H NMR spectrum of the Diels-Alder product (below) to H(exo), H(endo) and H(bridge,exo), H(bridge,endo). NOTE: exo/endo refers to the isomers and NOT the protons. 5.6 5.4 5.2 5.0 4.8 4.6 4.4 4.2 4.0 3.8 3.6 3.4 3.2 3.0 2.8 2.6