Acids and Bases Handout
advertisement

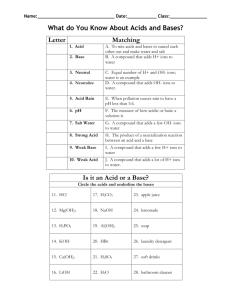
Name: Answer Key Date: ACIDS, BASES, & THE pH SCALE Pd: PART 1: ACIDS AND BASES Comparing Acids and Bases Acids Bases (Alkalines) Sour taste, leaves burning sensation Sharp bitter taste, feels slippery to touch Increase hydrogen ions (H+) in solution Decrease hydrogen ions (H+) in solution (Increase hydroxide ions (OH-) in solution) Example: HCl Example: NaOH Turns litmus (pH) paper to red Turns litmus (pH) paper to blue H+ + Cl- Na+ + OH- PART 2: THE pH SCALE The pH Scale is a measurement of how acidic or how basic a solution is. Compounds are acidic if they have a pH lower than 7. Compounds with a pH higher than 7 are basic or alkaline. Compounds with a pH of 7, are neutral. The scale goes from 0 to 14. 1 0 2 3 4 5 6 7 8 Increased concentration of H+ ions Decreased concentration of OH- ions pH below 7 = acids 9 10 11 12 13 Decreased concentration of H+ ions Increased concentration of OH- ions pH at 7 = neutral (pure water) pH above 7 = bases PART 3: BUFFERS A buffer is a weak acid or base that prevents sudden changes in pH. o Maintains homeostasis A buffer works by binding to H+ ions releasing H+ ions when the solution pH changes. Buffers bind to H+ ions when the concentration of H= ions in solution increases. Buffers release H+ ions when the concentration of H= ions in solution decreases. HCl H+ + Cl- NaOH 14 Na+ + OH- (The OH- will bind to H+ ions to form H2O – neutralization) ACIDS, BASES, & THE pH SCALE Biology Name: Date: Pd: PART 1: ACIDS AND BASES Comparing Acids and Bases Bases_____ (Alkalines_____) Acids_____ Sour_____ taste, leaves burning_____sensation Sharp bitter_____ taste, feels slippery_____ to touch Increase_____ hydrogen ions (H+) in solution (Increase_____ hydroxide ions (OH-) in solution) Decrease_____ hydrogen ions (H+) in solution (Increase_____ hydroxide ions (OH-) in solution) Example: HCl_____ Example: NaO_____H Turns litmus_____ (pH) paper to red_____ Turns litmus (pH) paper to blue_____ H+ + Cl-_________ Na+ + OH-________ PART 2: THE pH SCALE The pH Scale_______ is a measurement______________ of how acidic or how basic a solution is. Compounds________ are acidic_________ if they have a pH lower_______ than 7___. Compounds with a pH higher__________ than 7____ are basic__________ or alkaline____________. Compounds with a pH of 7_________, are neutral___________. 0 The scale goes from 0____ to 14____. 1 2 3 4 5 6 Increased_______ concentration of H+____ ions Decreased_______ concentration of OH-____ ions pH below 7 = acids____ 7 8 9 10 11 12 13 14 Decreased______ concentration of H+___ ions Increased______ concentration of OH-___ ions pH at 7 = neutral____ (pure_____ water_____) pH above 7 = bases_____ PART 3: BUFFERS A buffer_____ is a weak _____ _____ acid or _____ base that _____ prevents sudden _____ changes in pH. o Maintains homeostasis________ A buffer works by binding_____ to H+ ions releasing______ H+ ions when the solution pH changes. Buffers bind_____ to H+ ions when the concentration________ of H= ions in solution increases_____. NaOH________ Na+_____ + OH-_____ (The OH- will bind to H+ ions to form H2O – neutralization_____) Buffers release_____ H+ ions when the concentration________ of H= ions in solution decreases_____. HCl_____ _____ H+ + Cl-_____