Equilibrium Worksheet - Sayers-ONeill
advertisement

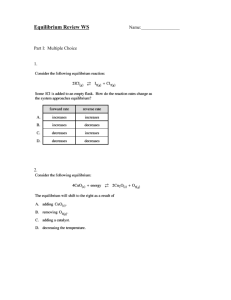
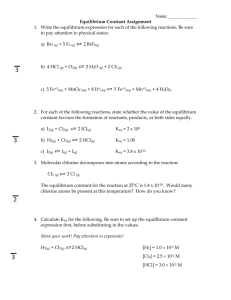
Equilibrium Worksheet Honors Chemistry Sayers-O’Neill Name: ____________________________ 1 Le Chatelier’s Principle Use this equation for questions 1 – 5. 2SO2(g) + O2(g) 2SO3(g) + heat Predict the direction of equilibrium shift (right, left, or no shift) if the following changes occur 1. O2 is added to the system. . 2. SO3 is added to the system. 3. The temperature of the system is increased. 4. A catalyst is added to the system. 5. The volume is decreased. Use this equation for questions 6 – 10 CO(g) + H2O(g) ↔ CO2(g) + H2(g) + heat 6. The addition of more H2O. 7. The removal of some H2. 8. Raising the temperature. 9. Addition of a catalyst. 10. Increasing the volume. Equilibrium Expressions 1. Write the equilibrium expression, Keq, for each of the following reactions: a.) 2 NO(g) + O2(g) ⇌2 NO2(g) f.) HC2H3O2(aq) + H2O(l)⇌ H3O+(aq) + C2H3O2-(aq) b.) 4 HCl(g) + O2(g) ⇌2 H2O(g) + 2 Cl2(g) g.) MgO(s) + CO2(g) ⇌ MgCO3(s) c.) NOCl(g)⇌NO(g) + ½ Cl2(g) h.) C(s) + CO2(g) + 2Cl2(g) ⇌ COCl2(g) d.) Fe3+(aq) + SCN-(aq)⇌FeSCN2+(aq) i.) Ca3(PO4)2(s) ⇌ 3 Ca2+(aq) + 2 PO43-(aq) 2+ e.) CaCl2(s) ⇌Ca (aq) + 2Cl (aq) j.) ZnO(s) + CO(g) ⇌ Zn(s) +CO2(g) 2. Classify the following equilibria as heterogeneous or homogeneous, and write an equilibrium expression for each. a) NH4NO2(s) ⇌N2(g) + 2 H2O(g) c) SO2(g) + ½ O2(g) ⇌ SO3(g) b) H2O(l) ⇌ H2O(g) d) S8(s) + 8 O2(g) ⇌ 8 SO2(g) Calculating Equilibrium Constants 1. The equilibrium constant for the equilibrium CO(g) + H2O(g) ⇌CO2(g) + H2(g) is 302 at 600K. What is the value of the equilibrium constant for the reverse reaction at the same temperature? 2. At the equilibrium point in the decomposition of phosphorus pentachloride to chlorine and phosphorus trichloride, the following concentrations are obtained: 0.010 mol/L PCl5, 0.15 mol/l PCl3 and 0.37 mol/L Cl2. Determine the Keq for the reaction. [5.55] 3. Gaseous dinitrogen tetroxide is placed in a flask and allowed to decompose to nitrogen dioxide and reach equilibrium at 100°C. At 100°C, the value of Keq is 0.212. If the concentration of dinitrogen tetroxide at equilibrium is 0.155 mol/L, what is the concentration of nitrogen dioxide at equilibrium? [0.181 M] 4. At 245°C, the equilibrium concentration of dinitrogen tetroxide gas is 6.38x10-3 mol/L and the total gas concentration is 1.23x10-2 mol/L. Determine the Keq for the decomposition of dinitrogen tetroxide gas to nitrogen dioxide gas at this temperature. [5.49 x 10-3] 5. The following reaction has Keq value of 85.0 at 460°C: SO2(g) + NO2(g) ⇌ NO(g) + SO3(g) If a mixture of sulfur dioxide and nitrogen dioxide is prepared, each with an initial concentration of 0.100 mol/L, calculate the equilibrium concentrations of nitrogen dioxide and nitrogen monoxide at this temperature. [NO2=9.8x10-3 M, NO=0.0902 M] 6. At 100°C the following reaction has a, Keq, of 2.2x10-10. COCl2(g) ⇌ CO(g) + Cl2(g) If 1.00 mol of phosgene, COCl2, is placed in a 10.0 L flask, calculate the [CO] at equilibrium. [4.7x10-6] 7. Six moles of SO2(g) and four moles of O2(g) are introduced into a 1.00 L reaction vessel and allowed to react to form SO3(g). At equilibrium, the vessel contains four moles of SO3(g). Calculate Keq for this reaction. [2.00] Equilibrium Worksheet Honors Chemistry Sayers-O’Neill Name: ____________________________ 2 8. Hydrogen and iodine gases react to form hydrogen iodide gas. If 6.00 mol of H2 and 3.00 mol of I2 are placed in a 3.00 L vessel and allowed to come to equilibrium at 250°C calculate the equilibrium concentrations of all species. The Keq for the reaction is 4.00 at 250 °C. [H2=1.33 M, I2=0.330 M, HI=1.33 M] Calculating and Applying Reaction Quotients 1. At 740°C, Keq = 0.0060 for the decomposition of calcium carbonate (CaCO3). Find Q and predict how the reaction will proceed if [CO2] = .0004M. CaCO3 (s) CaO (s) + CO2 (g) 2. Keq for the following reaction at 527°C is 5.10. If [CO] = 0.15 M, [H2O] = 0.25 M, [H2] = 0.42 M, and [CO2] = 0.37 M, calculate Q and determine how the reaction will proceed. CO (g) + H2O (g) H2 (g) + CO2 (g) 3. At 340°C, Keq = 0.064 for the reaction of rust with hydrogen gas. Given the [H2] = 0.45 M and [H2O] = 0.37 M, find Q and predict how the reaction will proceed.Fe2O3 (s) + 3H2 (g) 2Fe (s) + 3H2O (g) 4. The Keq for the following reaction at 2130°C is 0.0025. N2 (g) + O2 (g) 2NO (g) If [N2] = 0.81 M, [O2] = 0.75 M, and [NO] = 0.030 M, find Q and determine the direction in which the reaction will proceed. 5. At 500°C, the equilibrium constant for the following reaction is 0.080. N2 (g) + 3H2 (g) 2NH3 (g) Given that [NH3] = 0.0596 M, [N2] = 0.600 M, and [H2] = 0.420 M, find Q and predict how the reaction will proceed. 6. For the decomposition of antimony pentachloride (SbCl5) Keq = 0.0251. SbCl5 (g) SbCl3 (g) + Cl2 (g) What is the value of Q if [SbCl5] = 0.095 M, [SbCl3] = 0.020 M, and [Cl2] = 0.050 M? How will this reaction proceed? 7. At 1000°C, Keq = 1.0 x 10-13 for the following reaction. 2HF (g) H2 (g) + F2 (g) If [HF] = 23.0 M, [H2] = 0.540 M, and [F2] = 0.38 M, determine the value of Q; predict how the reaction will proceed. 8. At 1227 °C, Keq for the following reaction is 0.15. 2SO2 (g) + O2 (g) 2SO3 (g) If [SO2] = 0.344 M, [O2] = 0.172 M, and [SO3] = 0.056 M, find Q and determine how the reaction will proceed. Solubility Product and Common Ion Effect 1. What is the concentration of a saturated silver (I) acetate solution? Ksp(AgC2H3O2) = 1.94 x 10-3. 2. What is the concentration of a saturated lead chloride solution? Ksp(PbCl2) = 1.17 x 10-5. 3. A sample of solid strontium carbonate and pure water reaches equilibrium at 25 ºC. The concentration of strontium ion is 4.0 x 10-5 M. Calculate Ksp. 4. What are the concentrations at equilibrium of calcium and fluoride ions in a saturated solution of calcium fluoride? (Ksp = 3.9 x 10-11) 5. Consider the following equilibrium system: PbCl2 (s) Pb2+(aq) + 2Cl-(aq) Describe what happens to the solubility of PbCl2 when the following substances are added to the solution. Why? a) Pb(NO3)2 d) AgNO3 b) NaCl e) NaBr c) H2O 6. Consider the following equilibrium system: AgBr(s) Ag+(aq) + Br-(aq) Describe what happens to the solubility of AgBr(s) when the following substances are added to the solution. Why? a) Pb(NO3)2 c) NaCl b) AgNO3 d) NaBr 7. Explain why more Zn(OH)2 dissolves when 3 M HCl is added to a saturated solution of Zn(OH)2. Start by writing the correct equilibrium equation .