Chemistry 30 Unit 4 – Equilibrium Assignment
advertisement
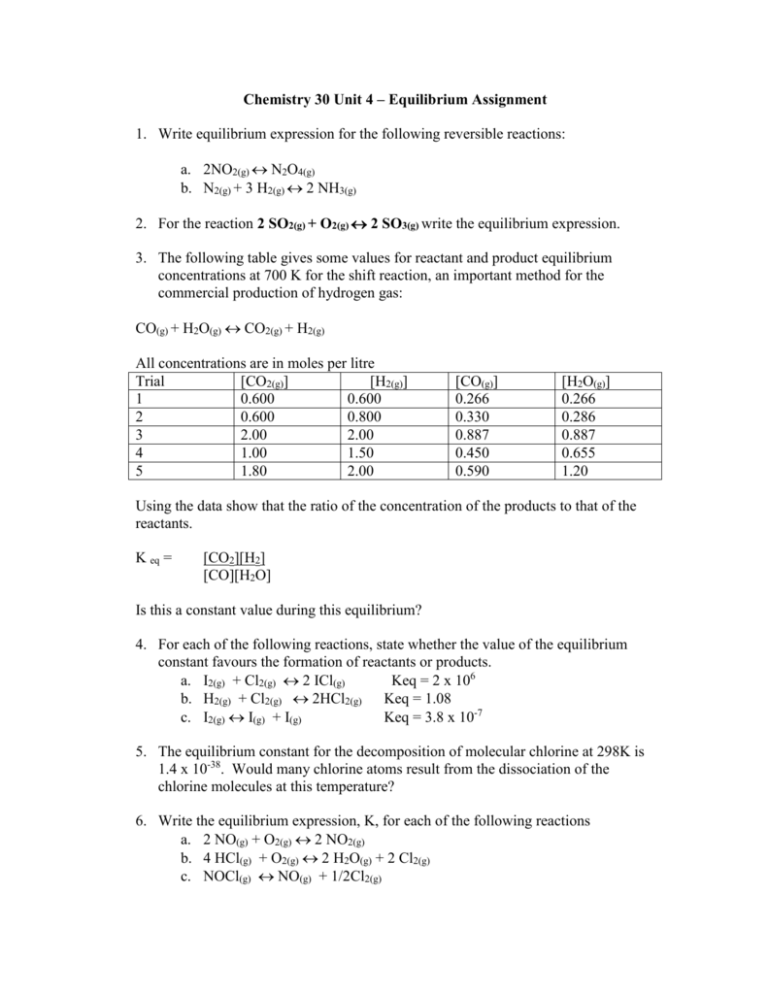
Chemistry 30 Unit 4 – Equilibrium Assignment 1. Write equilibrium expression for the following reversible reactions: a. 2NO2(g) N2O4(g) b. N2(g) + 3 H2(g) 2 NH3(g) 2. For the reaction 2 SO2(g) + O2(g) 2 SO3(g) write the equilibrium expression. 3. The following table gives some values for reactant and product equilibrium concentrations at 700 K for the shift reaction, an important method for the commercial production of hydrogen gas: CO(g) + H2O(g) CO2(g) + H2(g) All concentrations are in moles per litre Trial [CO2(g)] [H2(g)] 1 0.600 0.600 2 0.600 0.800 3 2.00 2.00 4 1.00 1.50 5 1.80 2.00 [CO(g)] 0.266 0.330 0.887 0.450 0.590 [H2O(g)] 0.266 0.286 0.887 0.655 1.20 Using the data show that the ratio of the concentration of the products to that of the reactants. K eq = [CO2][H2] [CO][H2O] Is this a constant value during this equilibrium? 4. For each of the following reactions, state whether the value of the equilibrium constant favours the formation of reactants or products. a. I2(g) + Cl2(g) 2 ICl(g) Keq = 2 x 106 b. H2(g) + Cl2(g) 2HCl2(g) Keq = 1.08 c. I2(g) I(g) + I(g) Keq = 3.8 x 10-7 5. The equilibrium constant for the decomposition of molecular chlorine at 298K is 1.4 x 10-38. Would many chlorine atoms result from the dissociation of the chlorine molecules at this temperature? 6. Write the equilibrium expression, K, for each of the following reactions a. 2 NO(g) + O2(g) 2 NO2(g) b. 4 HCl(g) + O2(g) 2 H2O(g) + 2 Cl2(g) c. NOCl(g) NO(g) + 1/2Cl2(g) 7. The equilibrium constant for the equilibrium CO(g) + H2O(g) CO2(g) + H2(g) Is 302 at 600K. What is the value of the equilibrium constant for the reverse reaction at the same temperature? 8. Classify the following equilibria as heterogeneous or homogeneous, and write an equilibrium expression for each. a. NH4NO2(s) N2(g) + 2 H2O(g) b. H2O(l) H2O(g) c. SO2(g) + ½ O2(g) SO3(g) d. S8(s) + 8 O2(g) 8 SO2(g)