Greek philosopher who coined the term atom
advertisement

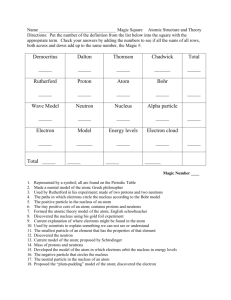
Memory Game Directions – Copy grid onto colored card stock. For homework or during class, students fill in the correct scientist for each description. (Don’t use marker or other writing utensil which can be seen through the back of the card stock). Put student in pairs. Have students check their work with their partner. Pair the pairs. There should be 4 students per group at this point. Have the pairs check their work. Any discrepancies can be decided by the teacher. The group will choose one card stock set that is correct and divide the work to cut it apart into individual cards. Each individual (or pairs of students) can play memory. The person or pair with the most matches wins. The uncut cardstock sheets can be used to check matches. Note some scientists are used more than once. After the memory game is finished the uncut cards can be used as flash cards. Simply cut the description and term/scientist out together and fold in half. I half extra cardstock copies for the person’s who’s cards were cut to make the memory game in case they want to have flash cards too. I find it is faster than tape. ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright Greek philosopher who coined the term atom. Discovered the neutrons in the nucleus of an atom. Greek philosopher who believed that matter was continuous. Said that electrons orbit the nucleus like the planets orbit the sun. Tried in vain to turn lead into gold. Discovered the element phosphorous. Studied gases and insisted that science be based upon experiments. Revived the idea of the atom and is considered father of atomic theory. Used a cathode ray tube to come up with his plum-in-thepudding model. Discovered the electron and determined the charge to mass ratio. Used his famous oil drop experiment to determine the charge on an electron. Used his famous gold foil experiment to discover the nucleus of the atom. Law that states that matter cannot be created or destroyed in ordinary chemical reactions. Law that states that a pure compound’s composition never changes. Law that states when 2 elements can combine in more than way, the ratio of their masses is a small whole number. Small positive particle found in the nucleus of an atom. Small neutral particle found in the nucleus of an atom. Small negative particle that orbits outside the nucleus of an atom. Said that the atom must be mostly empty space The tiny dense positive with a very small dense center of the atom. ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright positive center. ©2011 University of Illinois Board of Trustees • http://islcs.ncsa.illinois.edu/copyright