KIDNEY REGULATION OF OSMOLARITY
advertisement

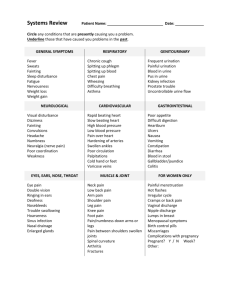
KIDNEY REGULATION OF OSMOLARITY Urinalysis.doc 29 April 2015 One of the kidney’s main functions is to regulate the osmolarity of body fluids at around 300mOsm per liter. In these experiments, the kidneys will be presented with excess water and/or salt load and the response will be monitored by measuring the volume and concentration of the urine produced. Overview of Lab: 1. Empty bladder 1-2 hrs before lab, and note the time. 2. When lab period begins, collect bladder contents, label as “control”, and 3. Drink test solution within 5 minutes. 4. Analyze “control” urine sample for volume and salt content. 5. Collect urine at 30 minute intervals to measure volume and salt content. 6. Determine the percentage of the water and/or salt intake excreted over 2.5 hours. Be able to answer these questions by the end of lab. a) What percentage of the original NaC1 and/or water load was excreted during the 2.5 hour interval following ingestion of the test liquid? Enter these values in the last row of the data table. (Exclude data from the “control” urine since this specimen was obtained prior to the ingestion of the test solution.) b) What is the osmolarity of the “strong salt” solution? Which hormone(s) is/are involved in the response to this solution? c) What is the osmolarity of the “dilute salt” solution? Which hormone(s) is/are involved in the response to this solution? d) Explain how the results illustrate the kidney’s handling of water and salt loads for each of the solutions consumed. Your explanation should include the roles of various hormones involved. e) What are the sources of error and variability in these experiments? 1. Drink plenty of water on the day of the experiment. Avoid eating or drinking caffeine or theophylline (all soft drinks, tea, chocolate). EMPTY YOUR BLADDER ONE OR TWO HOURS BEFORE THE LABORATORY BEGINS and RECORD THE EXACT TIME. Do not save this urine sample. It is not necessary to measure the volume of this sample. 2. Upon entering the laboratory, each student will take a urine collection bottle to the restroom and void into the bottle, emptying the bladder completely. Record the time. Take the specimen to the lab and label it as “control.” You will analyze this control sample after you have consumed the experimental solution. 3. Drink one of the following solutions in less than five minutes or even quicker if possible. You and your lab partners should agree on who will drink which solution. 800 ml of distilled water = plain water 800 ml of water plus 7 grams of NaCl = “dilute salt solution” 80 ml of water plus 7 grams of NaCl = “strong salt solution” These solutions are safe for humans with normal healthy kidneys. Do not drink one of the solutions containing salt if you have blood pressure problems or if you are on medications that might be affected by water or salt loading. You may elect not to participate in the experiment. Once you have consumed the test solution, you may not drink anything until the last urine sample is collected at about 150 minutes after drinking the test beverage. 1 4. Every 30 minutes after drinking the solution, each student will completely empty the bladder into a clean collection bottle. If unable to void, retain the urine in the bladder until the next 30 minute collection time, and adjust your calculations accordingly. 5. Analyze the urine from each collection starting with the first “control” sample, “ for: a) Volume: Measure the total volume with a graduated cylinder. We will convert this to ml/min excreted. (This is why it is important to know the time interval between specimen collections.) b) NaCl concentration: i) Pipette 1 ml of urine into a test tube. ii) Add one drop of 20% potassium chromate and mix thoroughly on Vortexer. iii) Titrate with 2.9% silver nitrate (0.171 Normal) solution dropwise from a burette while agitating the test tube on a Vortex mixer after each drop until a persistent color change is obtained. The end point of titration is a light pinkish brown. Disregard the formation of any precipitate; you are looking for a light brown solution. If you “overshoot” the endpoint of the titration (a dark reddish brown), discard the sample into the labeled waste container and start over. If your titration tube is approaching fullness, discard the sample, and start over using 500 microliters of urine, and the volume of Silver nitrate titrant should be doubled for your calculations. iv) Each ml of silver nitrate titrant represents 1 mg/ml of NaC1 in the urine (or 2 mg/ml if your urine sample was 500 microliters. v) Calculate: 1) the total grams of NaC1 in the urine collected at each 30 minute interval 2) the NaC1 concentration expressed as mg/ml of urine. 6. Record your data on the table on the next page. Calculations for the control (*) are complicated by the fact that the interval between the last urination before the lab and the first specimen collection may be different for each student. To make comparisons of NaC1 excretion before and after ingestion of the experimental solution, the NaC1 excreted during the pre-lab interval must be extrapolated to determine how much NaC1 would have been excreted in a 30 minute interval, so the special formulas apply only the first row in the table. Notation: tc = time interval between last urination before lab and collection of the control sample (in minutes) Vc = volume of control urine sample V1 = volume of first urine sample after ingestion of the test beverage. Uc = concentration of NaC1 in control sample of urine U1 = concentration of NaC1 in first urine sample after ingestion of the test beverage. Sample calculations: Time of last urination before lab: 12:48 pm Time of first urine collection during lab: 2:33 pm tc = time between 12:48 and 2:33 = 105 minutes (time over which control urine was produced) Let’s say 62 ml of urine was collected and the Na+ concentration of that control sample was 0.7 mg/ml (0.7 ml of silver nitrate titrant to reach the endpoint.) The first data row shows the calculations for the control sample. Subsequent rows shows the calculation for all the experimental samples that follow at 30 min intervals. 2 An example of how to calculate values for each cell in the data table. Time 0 (control sample) Volume of urine (ml) from Graduate Vc Urine flow (ml/min) Urine [NaC1] (mg/ml) = ml of titrant NaC1 Excreted in 30 min (mg) Vc/tc 62 ml 62ml/105min = Uc 0.7 mg/ml Uc*Vc*30 tc 12.4 mg U1*V1 0.5mg/ml*100ml = 50 mg U2*V2 0.59 ml/min 30 min V1 V1/30 100 ml 100 ml/30 min =3.3 U1 0.5 mg/ml ml/min 60 min V2 V2/30 U2 Time of last urination before lab period: ________________________ Test liquid (circle one): Pure water, Time 0 (control) 30 min 60 min 90 min 120 min 150 min Total Excreted in 2.5 hrs excluding the Control sample % of Intake Excreted excluding the Control Sample Volume (ml) Isotonic saline, or Urine Flow (ml/min) Hypertonic saline. Urine [NaC1] (mg/ml) * XXXXXXXX XXXX XX XX XXXXXXXX XXXX XX XX XXXXXXXX XXXX XX XX XXXXXXXX XXXX XX XX NaC1 Excreted in 30 min (mg) * * For the values in these cells, you must account for the time interval between your last urination prior to lab! See sample calculations in table at the bottom of the previous page. 3