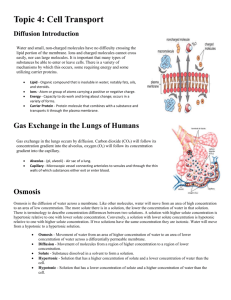
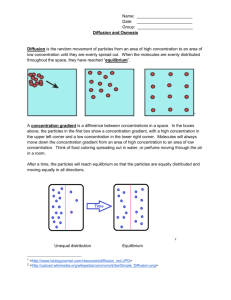
Diffusion, Osmosis
advertisement

BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY LAB: DIFFUSION, OSMOSIS, AND CELL MEMBRANE PERMEABILITY Lab format: this lab is delivered though a lab kit Relationship to theory: In the textbook (Reece et al., 9th ed.), this lab is related to the following unit: LEARNING OBJECTIVES At the end of this laboratory, you should be able to: 1. Describe the mechanism of diffusion at the molecular level. 2. List several factors that influence the rate of diffusion. 3. Describe the role of a selectively permeable membrane in relation to osmosis. 4. Describe hypertonic, hypotonic, and isotonic in terms of relative concentrations and movement across gradients. 5. Discuss the influence of the cell wall on osmotic behaviour in cells. 6. Apply principles of osmotic activity to medical, domestic, and environmental activities. 7. Describe the importance of cell size in relation to the passive movement of substances throughout it. 8. Describe the relationship between molecular weight, size, polarity, and partition coefficient in relation to membrane permeability and structure. PART I: DIFFUSION ACROSS A SEMIPERMEABLE MEMBRANE Introduction Homeostasis (the ability to adjust physiological processes to maintain internal equilibrium) in cells is achieved through regulated movement of materials through cytoplasm, across organelle membranes and across the plasma membrane. This regulated movement facilitates communication within the cell and between cytoplasm and the external environment. The cytoplasm and extracellular environment of the cell are aqueous solutions. Both are composed of water, which acts as a solvent or dissolving agent, and numerous organic and inorganic molecules, which are the solutes or dissolved substances. Organelle membranes and the plasma membrane are selectively permeable, allowing water to pass through freely, but regulating the movement of solutes. Some dissolved substances are actively moved across membranes, expending ATP (biological energy) to accomplish this movement. Other substances move passively across membranes, without the energy of ATP, but only if the membrane is permeable to those substances. Diffusion is the passive movement of water and selected solutes through the cell and cell membrane from an area where their concentration is higher to an area of lower concentration. If nothing hinders the movement, a solute will diffuse until it Creative Commons Attribution 3.0 Unported License 1 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY reaches equilibrium (i.e. the concentrations are equal). In addition to concentration, the directional movement of molecules can also depend on heat and pressure, though in biological systems we can assume that these factors will be equal, as temperature and pressure are relatively constant in living systems. Osmosis is the specific diffusion of water through a selectively permeable membrane from a region where it is highly concentrated to a region where it is less concentrated. The difference in concentration of water occurs if there is an unequal distribution of at least one dissolved substance. In this situation, the substance is called an osmotically active substance (OAS). For example, if a membrane that is impermeable to sucrose separates a solution of sucrose from distilled water, water will move through the membrane into the sucrose solution (where the water is in lower concentration). In this case, sucrose is the OAS. In terms of relative concentration, there are three terms that are used to describe solute concentration. A hypotonic solution is one that has a lower concentration of solutes relative to the environment. A hypertonic solution is one that has a higher solute concentration in relation to the surrounding environment. The third term, isotonic, describes a solute concentration that is equal to its surrounding environment. Remember, these terms describe the solute concentration. The net flow of water across a semi-permeable membrane is always from the area of lowest solute concentration to the area of highest solute concentration; therefore, water flows from the hypotonic solution to the hypertonic solution. The roots of these words may be of help in order to remember their meaning: Hyper means excess (think hyperactive=excess energy), hypo means below (hypodermic needle = below the skin), and iso means the same (isometric=balanced). Similar terms describe the osmolarity (solute concentration expressed as molarity) of a solution; hypoosmotic, hyperosmotic, and isosmotic. These terms relate the osmotic gradient, and should be thought of in terms of net water flow. The net flow of water is from the hypoosmotic solution through the membrane to the hyperosmotic solution. Isoosmotic solutions on either side of a membrane have no net flow between them. Either term can be used to describe a solution, but bear in mind “tonic” references the solute concentration and “osmotic” refers to the water concentration. Equipment 1 Raw egg 250 ml beaker 200ml acetic acid (white vinegar, at least 5%) 200ml glucose (light corn syrup) Scale (to measure to the nearest gram) Plastic wrap camera Creative Commons Attribution 3.0 Unported License 2 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY Procedure This lab will need to be monitored over a number of days. Be sure to read through the entire procedure before commencing. Photo-document the progress of your experiment at each of the following steps, and submit your photos with your report. 1. Weigh a raw egg. Measure the acetic acid in a graduated cylinder and transfer it to the beaker. Immerse the egg in the acid. Record egg mass and vinegar volume in table 2.1. Cover the beaker with plastic wrap and let it sit for 24 hours (or as long as it needs for the shell to be dissolved). Be aware that this is a chemical reaction, and the rate will be affected by temperature: if it is in a cool place, it may take longer. 2. Once the shell has dissolved and the membrane is visible, remove the egg from the acetic acid and weigh it. Be careful not to tear the membrane while handling the egg. Measure the remaining volume of the acetic acid in a graduated cylinder, and record the egg mass and vinegar volume in the table 2.1. Once the egg has been weighed, submerse it in 200ml of glucose (in a 250ml beaker): cover it, and leave for 24 hours. 3. Once the egg has been submerged for 24 hours in the glucose, remove it from the solution, weigh the egg and measure the volume of the glucose solution. Record in the table 2.1. Note any physical changes to the egg you observe. Once both the solution and egg have been measured, submerge the egg in 200ml of water. Cover and leave overnight. 4. After 24 hours, remove the egg from the water, weigh the egg and measure the water volume. Record your measurements in the table. Table 2.1: Egg mass and solution volumes Initial egg Initial vinegar mass volume Egg mass after Initial glucose vinegar soak volume Egg mass after Initial water glucose soak volume Egg mass after water soak Final Vinegar Volume Final Glucose volume Final water volume Discussion Questions 1. Define diffusion and osmosis in your own words, using examples. (2 marks) 2. The egg used here has a living semi-permeable membrane. Explain what this means, and how this demonstration models a cell. (3 marks) Creative Commons Attribution 3.0 Unported License 3 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY 3. Referring to the data collected in Table 2.1, describe the movement of materials across the membrane in each of the steps. It will be helpful to note that glucose is a relatively large molecule, and water molecules are relatively small and polarized. 4. Would you conclude the observed changes to be the result of diffusion or osmosis? Explain your answer. 5. In terms of cells, what might happen if the time elapsed in each of the steps was extended, or if the concentration of the solutions increased? 6. Consuming too much or too little water can have detrimental effects on human health. In paragraph form, investigate the side effects of these two scenarios at both the overall system level and the cellular level. (8 marks) PART II: SURFACE AREA TO VOLUME RATIO: THE IMPORTANCE OF CELL SIZE FOR DIFFUSION Introduction When cells reach a certain size, their rate of growth decreases. They will eventually stop growing. Each of these cells divides into two smaller cells, which begin to grow. What causes this? An easy way to investigate such questions is to build models. A model is often thought of as a small copy of something larger. Here we will be making a larger model of something small. When a cell becomes too large, it is no longer as efficient. It now has a greater volume and it takes much longer for materials that enter the cell membrane to reach the center of the cell. The typical eukaryotic cell is approximately 100 µm in diameter. Influence of size on surface area/volume ratios The purpose of this exercise is to see how the Surface Area to Volume ratio changes as an object gets larger. We will use a cube to serve as a model cell (or organism). To calculate the surface area-tovolume ratio, divide the surface area by the volume. Complete Table 2.2 for surface area volume ratios of a series of cubes of varying size. Creative Commons Attribution 3.0 Unported License 4 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY TABLE 2.2: SURFACE AREA TO VOLUME RATIO OF VARYING SIZED CUBES Length of a Surface Area Volume Surface/volume ratio side (mm) (mm2) (mm3) 1 2 3 4 5 6 7 8 9 10 Questions and Analysis: 1. Which cube has the greatest surface area? Volume? S/V ratio? (1 mark) 2. What happens to the surface area as the cubes get larger? What happens to the volume as the cubes get larger? What happens to the S/V ratio as the cubes get larger? (2 marks) 3. Proportionately, which grows faster - surface area or volume? Explain. (2 marks) 4. Using Excel, plot the following data on one graph: s/v ratio vs. cube size (length in mm); volume vs. cube size (length in mm); and surface area vs. cube size (length in mm). Include all appropriate labels. (5 marks) 5. Suggest some reasons why cells are small. (2 marks) 6. Explain why the rate of cell growth slows as a cell gets larger. (1 mark) 7. Explain why cells divide when they get large. (1 mark) PART III: CELL MEMBRANE PERMEABILITY AND THE PHYSICAL PROPERTIES OF SOLUTES Introduction This activity is concerned with some of the factors that determine the selective permeability of the plasma membrane of the red-blood cell. Water molecules pass through cell membranes by osmosis with relative ease moving through special channel proteins called aquaporins. These channels are ubiquitous in human cell membranes making Creative Commons Attribution 3.0 Unported License 5 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY them permeable to water. The permeability of solute molecules depends on a number of factors. Molecular size is important; small polar molecules pass through cell membranes more readily than larger molecules. Electrolytes generally pass through less readily than non-ionized polar molecules of similar size since ions carry electrical charges that attract and bind water molecules, forming larger hydrated spheres. Furthermore, active transport pumps in the cell membrane transport some ions against their concentration gradients (e.g. Na+, K+). Because of this, there is no net movement of these ions into or out of cells even when the membrane is permeable to them. Solubility in fat or non-polar solvents is also an important factor in determining cell membrane permeability. Non-polar compounds are highly soluble in fats but have very low solubility in water. Some polar compounds are freely soluble in water, but may also be soluble in oil if they have a number of nonpolar bonds (e.g. ethanol). Lipid soluble (non-polar) molecules pass through cell membranes by dissolving in, and diffusing through, the lipid portion of the membrane. Define the following terms (3 marks): Solution: Solute: Solvent: Solute particles move from one place to another (in solution) because of differences in their potential energy. Potential Energy is the energy objects have because of their position and internal structure in relation to each other. It is stored or inactive energy that has the potential (or capability) to do work but is not presently doing so. Differences in potential energy of substances in solution can be caused by differences in entropy (disorder), pressure, temperature, concentration of solute particles, etc. Solute particles move from a region where their potential energy is greater to a region where their potential energy is lower, regardless of the reason for the potential difference. In this example exercise, the gradient of potential energy of solute will be caused b y their gradient of concentration. As the concentration of solute particles increases, their potential energy increases, thus, solute particles will move from the region of higher solute particle concentration (where their potential energy is higher) to region of lower solute particle concentration (where their potential energy is lower) Like solute particles, water molecules also move from one place to another because of differences in their potential energy. This is usually referred to as the water potential. Water moves from a region where water potential is greater to a region where the water potential is lower, regardless the reason of Creative Commons Attribution 3.0 Unported License 6 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY for the water potential difference. In this example exercise, the gradient of water potential will also be caused by a gradient of concentration of solute particles. Water potential is affected by the concentration of dissolved particles of solutes and as the concentration of solute particles increases, the water potential decreases- but, why? The reason is, as the dissolved solute particles interrupt the ordered threedimensional interactions that normally occur between water molecules, entropy (disorder) of water will increase and thus water potential will decrease. We A B know that water molecules move from regions of high water potential to regions of lower water potential. This means that water moves from regions of low concentration of solute particles (low entropy and high water potential) to regions of high concentration of Permeable membrane solute particles (high entropy and low water potential). The concentration of solute particles is higher in region A than in region b. This means that the potential energy is higher in A than in B, thus the particles will move from A to B. This also means that the water potential in A is lower than in B, thus the water will move from B to A. Distinguish between the following terms with regards to the cell’s semi-permeable membrane (4 marks): 1. Diffusion: 2. Osmosis: 3. Penetrating solutes: 4. Non-penetrating solutes: Differentiate between the following terms (3 marks): 1. Molarity Creative Commons Attribution 3.0 Unported License 7 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY 2. Osmolarity 3. Tonicity A number of different ways exist to describe the concentration of a solution. The simplest way is to express it in weight of the solute per unit of volume of solution. For example, in grams or milligrams per litre of solution (150 mg/L of glucose) or per 100 ml of solution (15 mg/100 ml - or 15 mg%). However, it is frequently more advantageous to express concentration in terms of molarity. These measurements actually represent the number of molecules of solute per unit of volume of solution. One mole of any solute= 6.02 X 1023 molecules of solute. A one molar (1M) solution contains 1mole of solute molecules per litre of solution. Solutions with equal molarities have equal number of solute molecules, no matter what their solutes are. This means that a 2 molar (2M) glucose solution contains the same number of solute molecules as a 2 molar (2M) sodium chloride solution. One mole of solute is equal to the molecular weight of the solute in grams. The molecular weight of NaCl is 58.5 g and the molecular weight of glucose is 180 g-. In order to make a 2M glucose solution you will have to dissolve 360 g (2X180 g) of glucose in 1 liter of water. To make a 2M solution of NaCl, you have to dissolve only 117 g (2 x 58.5 g) of NaCl. A 1 molar solution is equivalent to the molecular weight of a molecule (in grams) dissolved in 1 liter of water Osmolarity represents the total concentration of all osmotically active solute particles in the solution. It is expressed in osmol/L (saying that a solution has an osmolarity of 1 osmol/L is equivalent to saying it has a total of 1 mole of osmotically active particles no matter what these particles are). When in solution, some solutes have molecules which dissociate into several particles. For example, the potassium chloride molecule (KCI) dissociates into two particles (K+, Cl-), the sodium chloride molecule (NaCl) also dissociates into two particles (Na+, Cl-), but the calcium chloride molecule (CaCl2) dissociates into three particles (Ca+, Cl-, Cl-). The osmolarity of solutions containing these solutes can be calculated from: OSMOLARITY = MOLARITY X THE NUMBER OF PARTICLES DISSOCIATED Creative Commons Attribution 3.0 Unported License 8 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY For example: The osmolarity of a 2M solution of NaCl is: 2 x2= 4 osmol/L The osmolarity of a 1M solution of CaCl2 is: 1 x 3= 3 osmol/L You can also use this equation to calculate the osmolarity of solutes that do not dissociate such as glucose. The osmolarity of a 4M solution of glucose is 4 x 1= 4 osmol/L. In the case of solutes that do not dissociate in solution (such as glucose, urea, glycerol, ...), the osmolarity of their solutions is equal to their molarity. Complete the following table (6 marks): TABLE 2.3: CALCULATING MOLARITY AND OSMOLARITY SOLUTE MOLARITY (mole/L) NaCl Urea EXPLANATION 4 3 CaCl2 CaCl2 OSMOLARITY (osmol/L) 0.3 0.2 TONICITY represents the ability of a solution to change the shape or turgidity of cells by altering their internal water volume (the ability of a solution to grab water from the cells). Every cell, including the red-blood cell, contains within the boundary of its cell membrane a certain number of non-penetrating solute particles (0.3 osmol/L) which will not diffuse out of the ceII. Red blood cells (RBC) in solution are often used to study the movement of water and particles across the cell membrane. Using red blood cells as an example, explain these terms by filling in the blanks in the following table. Creative Commons Attribution 3.0 Unported License 9 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY TABLE 2.4: MOVEMENT OF PARTICLES AND WATER ACROSS THE CELL MEMBRANE (fill in the blanks: 4 marks) Solution A is isotonic to red-blood cells Solution A is hyper-osmotic to red blood cells Solution A has a higher concentration of osmotically active solute particles than the redblood cells (osmolarity of the solution A is higher that the osmolarity of red blood cells Solution A is hypo-osmotic to red-blood cells Solution A has a lower concentration of osmotically active solute particles than the red-blood cells (osmolarity of the solution A is lower that the osmolarity of red blood cells Solution A is isotonic to red-blood cells Solution A is hypertonic to red-blood cells Water is drawn out of the cell by this solution and the red-blood cells shrink or crenate. Solution A is hypotonic to red-blood cells Water moves into the red-blood cells, the cells volume increases and cells can even burst. When red-blood cells rupture it is called hemolysis. Hemolysis of red-blood cells (the rupture of RBC) results when cells are placed in a hypotonic solution, as there is a net flux of water moving from the outside solution into the cells: inside the red-blood cell, the pressure exerted by the water on the cell’s membrane increases. The cells become distended, and when the elasticity of the cell membrane is reached, the cells’ membranes break (hemolysis). Crenation (shrinking) of RBC results when cells are placed in a hypertonic solution, where a net Creative Commons Attribution 3.0 Unported License 10 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY flux of water travelling across the membrane from inside the cell to the surrounding solution occurs. Inside the cells, the hydrostatic pressure decreases. The elastic cell membranes will collapse, and the cell will have a shrunken appearance. The membrane that surrounds the cells not only forms a boundary between the cell and its environment, but also serves to regulate the movement of materials from the cell to the environment and vice versa. Membrane permeability (ease with which it permits substances to pass through it) is an important factor in the normal functioning of a cell. In general, cell membranes are characterized as being selectively permeable. This means that a membrane will allow some substances (e .g. water) to readily pass across the membrane, while other substances (e.g. protein) are prevented from doing so. The ability of substances to cross a cell membrane depends: 1. 2. 3. 4. on their solubility in lipid; on their size; on their charge; on the presence of channels and transporters in the cell membrane. *Make a labelled diagram showing the molecular structure of the plasma membrane. Explain how each of the following classes of compounds passes through this membrane (8marks). 1. 2. 3. 4. 5. water and small polar solutes ionized solutes moderately large polar solutes (glucose) non-polar compounds (02, fatty acids, steroid hormones) very large molecules (proteins) Transport of small hydrophilic substances such as water, glycerol and urea, used in this example is accomplished via specific protein channels called aquaporins, which are found on almost all cells. Aquaporins are named based on their purpose in water transport. Many of the aquaporins are also in charge of moving other compounds like urea or glycerol across the membrane. In most cells these channels are not regulated; that is they are always open. Effect of Molecular Size on Permeability of the cell membrane The cell membrane is differentially permeable. Ionized solutes such as Cl-, inspite of their small molecular size, do not pass through rapidly. Moreover, active transport pumps for Na+ and Ca++ remove these ions from cytoplasm as fast as they enter cells. The cell membrane is more permeable to some of the unionized polar solutes used in this example. Creative Commons Attribution 3.0 Unported License 11 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY Relevant properties and formulae for the solutes used in this example are given in Table 2.5. Note that molecular size (diameter) roughly parallels molecular weight. All of these compounds are polar since they have either –OH (hydroxyl) or –NH2 (amino) groups. TABLE 2.5: PROPERTIES AND FORMULAE OF SOLUTES AND SOLVENTS Substance Molecular Weight (g) Molecular Diameter (Å) 3.6 Urea 60 Ethylene glycol 62 Glycerol 92 6.2 Glucose 180 8.6 Formula 3-D structure The following is a table of results that were observed by exposing red-blood cells (in suspension) to each of the substances in Table 2.5 (in solution). The time required for hemolysis (cell rupture) was recorded. TABLE 2.6: HEMOLYSIS TIME FOR RED BLOOD CELLS IN SOLUTES Substance Urea Ethylene glycol Glycerol glucose Mean Hemolysis Time (min:sec) 0:43 0:52 1:10 5:45 Creative Commons Attribution 3.0 Unported License 12 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY Questions 1. Using a spreadsheet program (e.g. Microsoft Excel), graph the molecular weight (horizontal axis) vs. mean times (vertical axis). Include all appropriate labelling and titles. (4 marks) 2. In a few sentences, interpret the graph in relation to cell membrane permeability and solute size. (4 marks) Effect of lipid solubility on permeability of the cell membrane Ions and water soluble molecules must pass through the pores in the membrane because they cannot dissolve in the central lipid layer of the membrane. On the other hand, lipid soluble molecule can dissolve in the lipid portion of the membrane and diffuse across easily. Many organic molecules contain both non-polar bonds, which grant lipid solubility, and polar bonds, which grant water solubility. Hydroxyl (-OH) and amino (-NH2) groups promote water solubility by virtue of the polar bonds between hydrogen and oxygen (in –OH groups) or between hydrogen and nitrogen (in –NH2 groups). On the other hand carbon-carbon or carbon-oxygen bonds are non-polar. When molecules contain both types of bonds they are soluble (to a greater or lesser extent) both in water and in lipids. The more polar bonds present, the greater is the water solubility, and the less soluble the compound is in lipids. The partition coefficient of a compound is a measure of its relative solubility in water and lipids. As the partition coefficient increases, fat solubility increases and water solubility decreases. Note in Table 2.7 that the partition coefficient increases as the number of polar hydroxyl groups decreases. PARTITION COEFFICIENT = SOLUBILITY IN OLIVE OIL/SOLUBILITY ON WATER Creative Commons Attribution 3.0 Unported License 13 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY TABLE 2.7: PARTITION COEFFICIENTS AND FORMULA Substance Molecular Weight (g) Partition Coefficient glycerol 92 0.00001 monoacetin 134 0.01 diacetin 176 0.1 triacetin 218 0.36 Formula CH2OH I CHOH I CH2OH CH2-O-COCH3 I CHOH I CH2OH CH2-O-COCH3 I CHOH I CH2-O-COCH3 CH2-O-COCH3 I CHO-COCH3 I CH2-O-COCH3 Questions 1. Based on the molecular weight of the substances in Table 2. , make a prediction about the time it would take for red-blood cells to hemolyse if added to each in solution. (2 marks) 2. Now reviewing the partition coefficients given, predict the hemolyses times for each substance. (2 marks) 3. Are the predictions in agreement? Discuss why you should not rely on a single factor to predict the permeability of the cell membrane to a specific substance. (4 marks) Creative Commons Attribution 3.0 Unported License 14 BIOLOGY SEMESTER ONE LAB: DIFFUSION, OSMOSIS, PERMEABILITY NANSLO Biology Core Units and Laboratory Experiments by the North American Network of Science Labs Online, a collaboration between WICHE, CCCS, and BCcampus is licensed under a Creative Commons Attribution 3.0 Unported License; based on a work at rwsl.nic.bc.ca. Funded by a grant from EDUCAUSE through the Next Generation Learning Challenges. Creative Commons Attribution 3.0 Unported License 15