Air, Water and Rocks Density Lab
advertisement

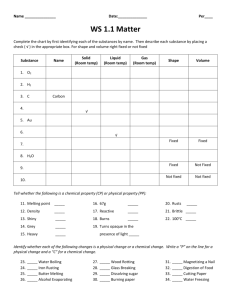
Air, Water and Rocks Density Lab Name: __________________ Period: 1 2 3 4 5 6 7 8 Air “floats” on the surface of the earth and the surface of the water. Water “floats” on rocks, and rocks sit on the bottom of it all. Water, air, and some rocks all sit on the top of the crust. This lab will help you realize why the air, water and rocks stay where they are. (Can you imagine a world where the air didn’t float on top?) After finding the density of air, water, and rocks, you will make predictions about why they organize themselves the way they do. 1- Air: The earth’s atmosphere is made of many different types of “air.” The main types are: - 78% Nitrogen - 21% Oxygen - <1% Carbon dioxide, argon, and other trace gases. To find the density of air, you will: a. Find the balloon’s mass by zeroing the scale with the empty balloon on it, blowing up the balloon, and then recording its mass as it reads on the scale. (Make sure its in grams!) b. Find it’s volume using water displacement in an upside-down graduated cylinder. c. Use the formula mass/volume to find density Mass of the air (in grams) Volume of the air (in mL) Density of the air (in g/mL) 2- Water: On our planet, water sinks in air and floats on rocks. Over 97% of the water on our planet is salt water. Today, you will be finding the density of fresh water using the following procedure: a. Find the mass of the fresh water by zeroing the scale with the empty graduated cylinder on it, carefully adding water to the graduated cylinder, and recording what the scale says for the mass of the water. (Make sure it’s in grams!) b. Find the volume of the water by recording where it comes to in the graduated cylinder. c. Use the formula mass/volume to find density. Mass of the water (in grams) Volume of the water in the graduated cylinder (in mL) Density of the water (in g/mL) 3- Rocks: Much of the earth that you walk on is made up of different types of rocks. To find the density of your rock, use the following procedure: a. Find the mass of your rock using a balance or scale b. Find the volume of your rock using water displacement in a graduated cylinder. c. Use the formula mass/volume to find density. Mass of the rock (in grams) Volume of the water in the graduated cylinder without the rock. (in mL) Volume of the water in the graduated cylinder with the rock. (in mL) Volume of the rock. (in cm3) Density of the rock (in g/cm3) Analysis and Questions: 1- What is the density of the: - Air: ____________ - Water: __________ - Rock: ___________ 2- How do these densities explain where the air, water, and rocks are found in our earth? __________________________________________________________________ __________________________________________________________________ 3- Draw an illustration of the earth and label the places you would expect to find air, water, and rocks. 4- Graph and label the densities of the air, water, and rocks on the density number line below: 0 1 2 3 4 5 6 7 8 9 10 I--------I--------I--------I--------I--------I--------I--------I--------I--------I----------I 5- Where on the number line do you think you would find metals like nickel or iron? __________________________________________________________________ Where in/on the Earth do you think you would find metals like nickel or iron? __________________________________________________________________ 6- Could we survive on an earth where things didn’t sort themselves out based on density? __________ Why or why not? ________________________________ __________________________________________________________________ __________________________________________________________________