ESTERIFICATION
advertisement

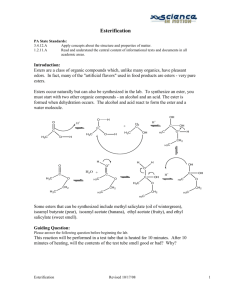
ESTERIFICATION Introduction: Esters are a class of organic compounds which, unlike many organics, have pleasant odors. In fact, many of the "artificial flavors" used in food products are esters - very pure esters. Esters occur naturally but can also be synthesized in the lab. To synthesize an ester, you must start with two other organic compounds - an alcohol and an acid. The ester is formed when dehydration occurs. The alcohol and acid react to form the ester and a water molecule. O O OH H + H + C+ C H3C O H H3C O H H+ H2 C H3C C H3C OH OH O+ H CH 2 H3C H H2O + C H3C H O+ O O H3C H O C C O H3C CH 2 H3C OH + OH C H+ H3C O O CH 2 CH 2 CH 2 H3C H3C H3C Some esters that can be synthesized include methyl salicylate (oil of wintergreen), isoamyl butyrate (pear), isoamyl acetate (banana), ethyl acetate (fruity), and ethyl salicylate (sweet smell). Purpose: The purpose of this experiment is to synthesize and identify four esters. Equipment/Materials: hot plate 250 mL beaker styrofoam cups (4 per group) Beral pipet (1 per group) microscale thermometers labelling tape OH Dowex 50 x 2 - 100 ion-exchange resin glacial acetic acid (20 mL) salicylic acid (10 g) isoamyl alcohol (15 mL) methanol (20 mL) ethanol (15mL) Safety: Always wear safety glasses and an apron. Keep all reactants away from flames. Do not smell chemicals by placing your nose at the bottle’s opening; waft the smell toward your nose with your hand. Never eat or drink in the lab. Procedure: 1. Heat water in a beaker on a hotplate near a wall. Place a microscale thermometer in the water. Monitor the temperature – it should stay between 70 – 80 oC. 2. Label 4 microscale test tubes A – D at the top with labeling tape. The test tubes may be placed in microscale Erlenmeyer flasks, which will act as stands for the test tubes. 3. Use the microspatula to place a small amount of ion-exchange resin (about equal to two garden peas) into the four test tubes. 4. Place the following in the test tube labeled “A”: 10 drops of isoamyl alcohol 10 drops of glacial acetic acid 5. Place the following in the test tube labeled “B”: 10 drops of ethanol 10 drops of glacial acetic acid 6. Place the following in the test tube labeled “C”: salicylic acid – a similar amount to the resin enough methanol to dissolve the acid (about 25 drops) 7. Place the following in the test tube labeled “D”: salicylic acid – a similar amount to the resin 10 drops of ethanol 8. Place all four test tubes in the water bath on the hot plate, and allow them to react for 10 minutes. Be sure that the test tubes are aimed at the wall. (It is not necessary to put the Erlenmeyer flasks in the water.) 9. After 10 minutes, CAUTIOUSLY smell the odor of each. Remember to waft the smell towards your nose. What do you smell? Record on the data table. 10. Pour the liquid contents of tube A into a clean styrofoam cup. Swirl the cup one or two times, then immediately pour the excess reactants into a sink, and rinse with a small amount of water. Smell the inside of the cup. Record what you smell. 11. Repeat step 10 for the examination of the other three esters. 12. Thoroughly rinse out each of the test tubes and cups. Data Table: TUBE SMELL INITIAL SMELL AFTER RINSING A __________________________ ________________________ B __________________________ ________________________ C __________________________ ________________________ D __________________________ ________________________ Questions: 1. Tube A contained the following: ___________________smell or ___________________ester or ___________________ester or ___________________ester or ___________________ester 2. Tube B contained the following: ___________________smell 3. Tube C contained the following: ___________________smell 4. Tube D contained the following: ___________________smell 5. How did the smell of each ester change after rinsing with water? 6. What name would you give to an orange-smelling ester formed by adding octanol to acetic acid? ESTERIFICATION TEACHER NOTES STANDARDS MET: 3.4.7.A - Describe concepts about the structure and properties of matter. Describe and conduct experiments that identify chemical and physical properties. 3.2.10.C – Apply the elements of scientific inquiry to solve problems. Conduct a multiple step experiment. 3.4.10.A – Explain concepts about the structure and properties of matter. Explain the formation of compounds and their resulting properties using bonding theories (ionic and covalent). Understand that carbon can form several types of compounds. 3.7.12.A – Apply advanced tools, materials and techniques to answer complex questions. Demonstrate the safe use ofcomplex tools and machines within their specifications. Evaluate and use technological resources to solve complex multistep problems. Lab Time: 45 minutes Answers to Questions: 1. Tube 1 contained the following: ___banana__________smell or __isoamyl acetate____ester or __ethyl acetate_____ester or _methyl salicylate__ester or __ethyl salicylate__ester 2. Tube 2 contained the following: _____fruity________smell 3. Tube 3 contained the following: ____wintergreen____smell 4. Tube 4 contained the following: ___sweet___________smell 5. How did the smell of each ester change after rinsing with water? Smell should have become stronger because “harsh” un-reacted chemicals were washed away. 6. What name would you give to an orange-smelling ester formed by adding octanol to acetic acid? octyl acetate Considerations: This lab is a version of one developed by Brother Carmen Ciardullo (Micro Action Chemistry , Flinn Scientific inc., 1990). This book contains a wealth of microscale experiments and is suggested for any teacher who is considering incorporating microscale labs into a chemistry class. One change from the original procedure is to rinse the reactants in a cup and then rinse one time with water. This seems to allow the esters to be smelled much easier due to the increased surface area and the removal of harsh “masking” chemicals. Students will be using concentrated acids so caution must be maintained at all times. Caution should be taken while heating the tubes. Students can also make other esters using this equipment but, as with any lab, the instructor should first experiment to determine if results are acceptable and safety is maintained. The production of the esters can be verified using gas chromatography (see Verification of Esterification). Last updated 11-02.