Key to ws13.2
advertisement

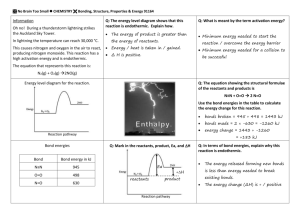
Chapter 13 Worksheet 2 (ws13.2) Collision Theory, Elementary Reactions, Energy Diagrams, Mechanisms, Catalysis 1. For a chemical reaction to occur, reactants must collide. However, usually only a small fraction of collisions actually result in a successful reaction. Name two reasons for this. a. Reactants must collide with sufficient force in order to provide the energy required to break bonds. This is called “activation energy”. b. Reactants must collide in a particular orientation for a reaction to occur. 2. List 3 ways to increase the rate of a reaction and explain why they work based on your answers to question 1. a. Increase the concentrations of reactants. This would increase the frequency of collisions. Similarly, increasing the surface area of a solid reactant (by breaking it up into smaller pieces) would increase the frequency of collisions. b. Increase the temperature. This would increase both the frequency and the force of collisions. c. Add a catalyst. Catalysts reduce the energy required to start a reaction (activation energy) so the molecules don’t have to collide as hard. Solid (heterogeneous) catalysts provide a surface on which the reaction can occur. This increases the probability, and thus the frequency, of collisions. Biological catalysts, called enzymes, can hold the reactants near each other in the optimal orientation for a reaction to occur. 3. On a single pair of axes, sketch two Maxwell-Boltzmann distributions that represent the distributions of kinetic energies among molecules of an ideal gas at two different temperatures. 1 4. Define elementary reaction and explain the difference between unimolecular, bimolecular, and termolecular elementary reactions. Elementary reaction: A reaction that occurs during the course of a single collision. Not a whole lot can happen during one collision. Typically no more than one bond is broken and one bond is formed. Unimolecular reaction - elementary reaction involving only a single molecule (How is this possible? See below.) Bimolecular reaction - elementary reaction involving the collision of two molecules Termolecular reaction - elementary reaction involving the collision of three molecules (These are rare. Do you understand why?) 5. If reactants must collide in order to react, how can a reaction be unimolecular? A reaction is unimolecular if it doesn’t matter what the reactant collides with. It could collide with another molecule in the reaction, it could collide with the solvent molecules, or it could collide with the walls of the container. Decompositions and rearrangements (isomerizations) are usually unimolecular. The reactant simply has to collide with something hard enough to break a bond. 6. Why are termolecular (or reactions with higher molecularity) unlikely? It is unlikely that 3 or more molecules could all come together in space at the same moment. It is even less likely that they would all have the correct orientation required for reaction to occur. Termolecular reactions do occur but they are very slow. 2 7. Draw and label an energy diagram that shows how the potential energy of the molecules in a reaction changes during the course of an elementary reaction. An energy diagram shows how the potential energy changes as reactants are converted to products. The x-axis represents the progress of the reaction. (You can think of the x-axis as representing time.) The highest energy species produced during a reaction is called the transition state (or activated complex). The energy required to produce the activated complex (height of the hill) is called the activation energy (Ea). The difference between the potential energies of the products and reactants (E) is approximately the same as H if the reaction is done at constant temperature and pressure and if only “PV” work is done by the reaction. PV work is work done by the expansion or compression of gases. (See proof in the optional material at the end of this worksheet.) 8. Very few reactions occur in a single elementary step. Instead, they occur through a series of elementary steps called the reaction mechanism. The elementary steps in a multistep mechanism must always add to give the chemical equation of the overall process. For each of the following mechanisms, determine the net reaction and identify the reaction intermediates. a. NO2(g) + NO2(g) → NO3(g) + NO(g) Intermediates: NO3(g) NO3(g) + CO(g) → NO2(g) + CO2(g) Net: NO2(g) + CO(g) → NO(g) + CO2(g) b. H2(g) + ICl(g) → HClI(g) + H(g) H(g) + ICl(g) → HCl(g) + I(g) HClI(g) → HCl(g) + I(g) I(g) + I(g) → I2(g) Net: H2(g) + 2 ICl(g) → 2 HCl (g) + I2(g) Intermediates: HClI(g), H(g), I(g) 3 9. The rate of a reaction is equal to the rate of the slowest step in the mechanism. The slowest step is called the rate determining step or the rate limiting step. Consider the mechanism in question 8b. Which of the steps would you guess is the slowest? The fastest? Predict whether each step is endothermic or exothermic. Step 1: Bimolecular; Break two bonds and form one bond; Probably endothermic Step 2: Bimolecular; Break one bond and form one bond; Endothermic if an HCl bond is stronger than an ICl bond. Step 3. Unimolecular; Break one bond; Endothermic Step 4: Bimolecular; Form one bond; Exothermic Most likely order from slowest to fastest: step 1, step 2, step 4, step 3 10. What is the relationship between the activation energy for an elementary reaction and the rate of that reaction? As activation energy increases, the rate decreases because fewer collisions provide sufficient energy to get over the activation energy barrier. 11. Draw an energy diagram that represents the mechanism in question 8b. Assume that the reaction occurs at constant temperature and pressure and that the net reaction is exothermic. 4 12. A catalyst speeds up a chemical reaction without being consumed. List 3 ways that a catalyst can do this. A catalyst participates in the chemical reaction and provides an alternative mechanism whose slowest step has a lower activation energy. In general, a catalyst can lower the energy of (stabilize) the transition state or it can raise the energy of (destabilize) the reactants: a. The catalyzed reaction pathway can produce a new transition state that is more stable than the original transition state. b. A catalyst can stabilize the transition state by binding tightly to it (enzymes can do this) c. A catalyst can destabilize the reactants by binding to them in such a way that the bonds are stressed (enzymes and heterogenous catalysts can do this). 13. For the mechanism below, write the net reaction and identify all intermediates and catalysts. Ce4+ + Mn2+ → Ce3+ + Mn3+ Ce4+ + Mn3+ → Ce3+ + Mn4+ Mn4+ + Tl+ → Mn2+ + Tl3+ . Net: Intermediates: Mn3+, Mn4+ Catalysts: Mn2+ 2Ce4+ + Tl+ → 2Ce3+ + Tl3+ 14. Go back to your answer to question 11 and draw a second energy diagram that represents the same reaction in the presence of a catalyst. Assume that the catalyst changes the mechanism such that the rate determining step becomes two steps and that the rest of the mechanism remains unchanged. 15. Draw an energy diagram for an explosive reaction. (Assume a single step mechanism) 5 Supplementary Material (Optional) If a reaction is performed at constant temperature and pressure and if only PV work is done, then Epot ≈ H. Epot is the difference in the potential energies of the products and reactants.) This is demonstrated below. The potential energy of a molecule is the energy stored in the chemical bonds. A molecule with strong bonds is very stable so it has low potential energy. A molecule with weak bonds is unstable and it has high potential energy. The total energy content of a molecule (E) is the sum of its potential energy (Epot) and kinetic energy (Ekin): E = Epot + Ekin If temperature is held constant: E = Epot (kinetic energy doesn’t change) E = q + w (where q is heat absorbed by the system and w is work done on the system) At constant pressure and if only “PV” work (expansion or compression of gases) is done: E = qp - PV = H - PV (recall that, by definition, H = qp where qp is heat absorbed at constant pressure) Why the negative sign? Recall that positive work is work done on the system. This occurs when gases are compressed. When gases are compressed, V is negative so the work term is positive as expected. If pressure is not too large and the volume change is small: E = Epot ≈ H If no gases are involved in a chemical reaction thenV ≈ 0 and Epot = H 6