Preparation and transformation of competent bacteria: Calcium
advertisement

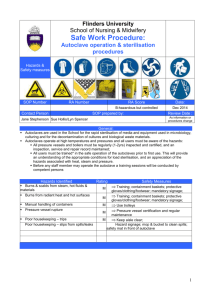
533571216 October 7, 2004 Boss4 Appendix B: Commonly used techniques Sterilizing in molecular biology. Most solutions we work with in molecular biology are sterile. The need for sterile growth media is somewhat obvious; after all, we are trying to grow very specific organisms, not just whatever falls out of the air. Many other solutions we use in molecular biology can also be nice substrates for microbial growth. When we isolation plasmid, or run a gel, we prefer to look at DNA from the bacteria we were using as a cloning vehicle, not the DNA of bacteria or fungi that are just lying around the lab. Even small amounts of contamination can cause big problems, especially if you will be using amplification techniques, such as PCR, downstream. Keeping all solutions sterile means you can make larger batches of solutions to use over longer periods of time. Otherwise, you will find yourself preparing fresh solutions more often then you would like. Additionally, before disposal we are required to decontaminate the following: all genetically modified organisms, anything contaminated with such organisms, or nucleic acids from such organisms. In other words, pretty much anything we work with should be treated as a biohazardous waste. The easiest and most affective way to decontaminate most waste is by autoclaving. Exceptions include human and animal tissue, which should be incinerated for disposal, and items contaminated by volatile organics, such as phenol, or chloroform, which should go to a certified chemical disposal plant. Sterilizing by autoclaving. Autoclaving, sometimes called steam sterilization, is the use of pressurized steam to kill infectious agents and denature proteins. Water is heated to temperatures above its boiling point (to a minimum of 120 C) by holding it in a pressurized chamber. This kind of "wet heat" is considered the most dependable method of sterilizing laboratory equipment and decontaminating biohazardous waste. Autoclaves do not remove chemical contamination. In fact autoclaving some chemicals can lead to production of dangerous fumes. Packaging of items, or liquids to be sterilized, must permit heat (steam) penetration, and prevent pressure differentials that could result in breakage. This may be accomplished by using techniques such as: Loosening screw caps or using self venting caps Capping open containers for sterilization with aluminum foil Opening plastic bags slightly prior to loading them into the autoclave. Do not place sealed containers in an autoclave! Additionally, make sure any containers you put in the autoclave can handle the heat and pressure. For glass, pyrex is a good choice, for plastic PP (polypropylene) or polycarbonate (PC) are the best choices. Page 1 of 2 533571216 October 7, 2004 Boss4 Autoclaving liquids 1. Loosely set caps on bottles, or cover openings with foil or foam plugs. 2. Autoclave for 20 min at 120 C and 15 lb/sq. in. using a slow exhaust (using fast exhaust can result in boiling over of superheated solutions). Longer times for autoclaving may be required if working with large volumes. 3. Only after solutions have completely cooled, tighten the lids. Decontaminating solutions containing ethidium bromide. There are a variety of ways to remove ethidium bromide from solution, primarily gel running buffers. All of them involve binding up the ethidium bromide with some type of solid. Once decontaminate, the remaining solution can be safely poured down the drain. The contaminated solid material is sent to a chemical waste disposal plant for further treatment Examples of solids which will bind ethidium bromide include: activated charcoal certain resins Autoclaving plastics, tips in tip boxes, glass etc. 1. Wrap plastics, glass etc. in foil, PP bags, or place in appropriate sized plastic or pyrex containers. 2. Loosely set caps on any containers. 3. Autoclave for 20 min at 120 C and 15 lb/sq. in. Inert materials can be exhausted either fast or slow. Drying cycles are optional, if available they will remove most of the condensed water from the items. 4. Only after containers have completely cooled, tighten the lids. How to filter sterilize Solutions that contain heat-labile components must be filter-sterilized. Such solutions include many proteins, vitamins, amino acids, carbohydrates and most antibiotics. To filter sterilize small volumes (10 ml or less) use a filter which can be fitted on the end of a syringe. For larger volumes use sterile filters which can be fitted onto a bottle top. Porosity of the filter membrane should be no larger than 0.2 microns (µm). Collect the filtered solution in sterile containers. Page 2 of 2