E. coli Competent Cell Prep
advertisement

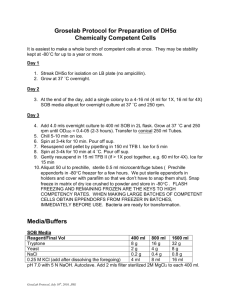
E. coli Competent Cell Prep For HB101, DH5, XL1-Blue (Molecular Cloning Manual 2nd ed. 1989. vol. 1) 1. Inoculate 10 ml O/N culture from a plate or frozen stock. From the O/N culture, inoculate 0.5 ml into 50 ml LB (in a sterile 250 ml flask). Incubate at 37oC until the OD600=0.6. This will take 2 - 4 hrs. 2. Cool the cultures by storing on ice for approx. 10 min. Harvest by centrifugation in a sterile tube at 4000 rpm/10min/4oC. 3. Decant the medium from the cell pellets. Resuspend each pellet in ice-cold 0.1 M CaCl2 or TFB (10 ml/pellet from 50 ml culture). Incubate on ice for 10 min. 4. Centrifuge cells at 4000 rpm/10 min. at 4oC. Gently resuspend cells in ice-cold CaCl2 or TFB (2 ml/50 ml original culture) and store at 4oC. Notes The use of TFB may increase transformation efficiency. The efficiency of transformation increases during the first 12-24 hours of storage, then decreases to the original level. Glycerol (final concentration = 15%) can be added to the cells for storage at -70oC, although the transformation efficiency will decrease. Transformation (Adapted from a Stratagene protocol accompanying their Epicurian Coli SCS1 (Cat #200231) competent cells) 1. Aliquot 100 ul competent cells into a pre-chilled 15 ml Falcon 2059 polypropylene tube (Or equivalent; other tubes may be degraded by the BME. In addition, the heat pulse step is critical and has been calculated using the heat transfer properties of falcon tubes. A defined "window" of 45 - 50 seconds is necessary for optimal transformation efficiencies). If competent cells have been frozen, thaw on ice and mix gently before transferring to the falcon tubes.. 2. Add 3.4 ul of a fresh 1:10 dilution of BME (B-Mercaptoethanol) diluted in high-quality water, to the 200 ul cells. 3. Swirl gently. Incubate on ice for 10 minutes, swirling gently every 2 minutes. 4. Add 0.1 - 50 ng DNA to the cells and swirl gently. Incubate on ice for 30 minutes. Heat pulse for 45 seconds (this step is very critical; see step 2) in a 42oC water bath. Incubate on ice for 2 minutes. 5. Add 800 ul SOC broth (preheated to 42oC) and outgrow the cells for one hour at 37oC, shaking gently. 6. Plate an appropriate amount of cells on LB agar plates (with the appropriate antibiotic). Incubate the plates at 37oC O/N. Notes To determine the transformation efficiency, transform with 1 ng supercoiled plasmid DNA and plate 10 and 100 ul. For subcloning, concentrate the cells after the outgrowth (centrifuge at 4K/2 min in a microfuge and resuspend in 100 ul SOC). Plate 10 ul and 90 ul. Buffers and Media 0.1 M CaCl2 (500 ml) 7.35 g CaCl2•2H20 Autoclave 1 M MES (2-[N-morpholine]ethanesulfonic acid) Dissolve 19.52 g MES in 80 ml ddH2O Adjust pH to 6.3 with 5 M KOH and bring to final volume of 100 ml Filter sterilize through 0.45 micron filter, and store at -20oC in 10 ml aliquots TFB Reagent 1 M MES (pH 6.3) MnCl2•4H2O CaCl2•2H2O KCl Hexamminecobalt chloride Amount/litre 10 ml 8.91 g 1.47 g 7.46 g 0.8 g Mix all components in 800 ml ddH20 Adjust volume to 1 litre and filter sterilize Freeze at -20oC or store at 4oC in 40 ml aliquots The pH of the solution should be between 6.0 and 6.1 SOC Broth (1 litre) 20 g Bacto-Tryptone 5 g Yeast Extract 0.5 g NaCl Autoclave Before use add: (per 100 ml SOB) 1 ml MgCl2/MgSO4 2 ml sterile 20% glucose MgCl2/MgSO4 (100 ml) 12 g MgSO4 9.5 g MgCl2. Filter sterilize. 20% Glucose (100 ml) 20 g glucose (dextrose) Autoclave or filter sterilize Final concentration 10 mM 45 mM 10 mM 100 mM 3 mM