Protocol
advertisement

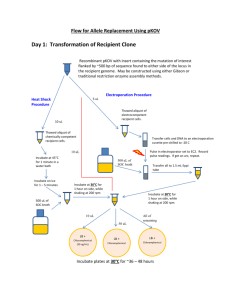
LR Cloning LR reaction 1. Add the following components to a PCR tube or 96-well plate at room temperature and mix: Entry clone (stock solution 150ng/ul) Destination vector (stock solution 150ng/ul) TE buffer, pH8.0 or water 1ul 1ul 2.6ul 2. Thaw on ice the LR clonaseTM II enzyme mix (stored at -80oC) for about 2 minutes. Vortex the LR clonaseTM II enzyme mix briefly twice (2 seconds each time). 3. To each sample add 0.4ul of LR clonaseTM II enzyme mix to the reaction and mix briefly twice. Microcentrifuge briefly. 4. Return LR clonaseTM II enzyme mix to -80oC storage. 5. Incubate reactions at 25oC for 2 hours or overnight in a PCR machine. Transformation 1. Defrost competent cells on ice (approx. 10 mins). 2. Add 1ul of each LR reaction to a 1.5ml tube containing 10ul of competent cells. 3. Incubate on ice for 30 minutes. 4. Heat shock at 42oC for 30 seconds. 5. Incubate on ice for 2 minutes. 6. Add 250ul of SOC medium and incubate at 37oC for 1 hour with shaking. 7. Plate 100ul of cells onto selective LB agar plates. 8. Incubate plates at 37oC overnight. 9. Remaining LR reaction can be stored at -20oC in case transformations need to be repeated. Checking colonies 1. Patch 10 colonies from each transformation onto selective LB agar plates for the donor vector and also selective LB agar plates for the destination vector and incubate at 37oC overnight. 2. Identify colonies capable of growth on the selective LB agar plates for the destination vector only. 3. Perform colony PCR for 8 positive colonies for each clone (see separate protocol). 4. Perform minipreps for 2 positive colonies for each clone (see separate protocol. 5. Sequence to confirm clone