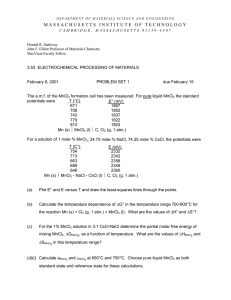
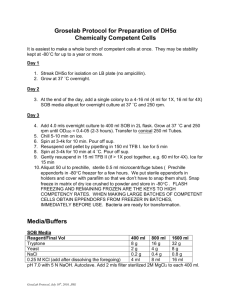
COMPETENT CELL PREPARATION
advertisement

Competent Cell Preparation 1 of 1 Updated 04/03/11 by y.z. COMPETENT CELL PREPARATION (Inoue et al. Gene, 96: 23-28, 1990) 1) Frozen stock of E. coli cells were thawed, streaked on an LB-agar plate and cultured O/N at 37˚C. 2) Ten to twelve large colonies were inoculated to 250 ml SOB-media in a 2 L flask (LB gives lower efficiency) and grown to an A600 of 0.6 at 18˚C, with vigorous shaking (200-250 rpm). 3) Place flask on ice for 10 min. Transfer the culture to centrifuge bottle and spin at 2500xg (3000 rpm Beckman J-6B) for 10 min at +4˚C. 4) Resuspend the pellet in 80 ml ice-cold TB and incubate in ice bath for 10 min and spin down as above. 5) Resuspend the pellet in 20 ml of TB and add DMSO (not cold) with gentle swirling to a final concentration of 7%. Incubate on ice for 10 minutes. 6) Aliquot the cell suspension into cold cryotubes and freeze in liquid nitrogen. SOB: Per liter Per 250 ml tryptone 20 g 5g yeast extract 5g 1.25 g NaCl 0.6 g 0.15 g KCl 0.5 g 0.125 g -----------------------------------------------------------------------------MgCl2 10 mM 20 ml 5 ml (add just before using) MgSO4 10 mM i) Disolve the tryptone, yeast extract, sodium choride, and potassium chloride in pure water. Sterilize by autoclaving. ii) Prepare a 2M stock of Mg2+, consisting of 0.5M MgCl2 and 0.5M MgSO4. Sterilize by filtration 0.22 μm. iii) Just prior to use, combine the medium with 20 ml of the magnesium stock. TB: Per 100 ml 10 mM Hepes 0.238 HEPES 15 mM CaCl2 0.17g 250 mM KCl 1.86g -----------------------------------------------------------55 mM MnCl2 1.09g i) Mix all components, except for MnCl2 (all salts were added as solids) and adjust the pH with KOH to 6.7. ii) Dissolve MnCl2 and sterilize by filtration (pre-rinsed 0.45 μm) and store at +4˚C.