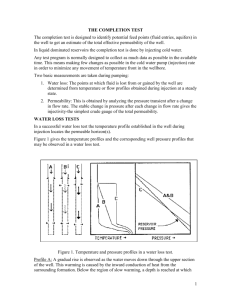
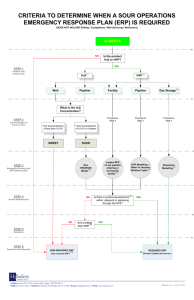
Robert A. Marriott - University of Calgary
advertisement

Robert A. Marriott Biographical Abstract Dr. Marriott is an experimental thermodynamicist with research interests in high pressure sour gas properties and sulfur chemistry. He completed his MSc at the University of Lethbridge studying highpressure aqueous chemistry and his PhD at Dalhousie University studying solid state physical chemistry / materials science. For the past five years, his research has focused on understanding elemental sulfur deposition during the production of sour gas, sulfur solvent properties, water content for sour gas mixtures, sulfur degassing mechanisms, reservoir mineral interactions, high-pressure adsorption interaction and pVT properties for SO2 and H2S mixtures. He routinely communicates fundamental research and concepts to industry professionals world-wide through ASRL industrial chemistry courses and international conferences. In addition to maintaining a lead scientist role for ASRL’s high pressure research, Dr. Marriott lectures as a sessional instructor within the Department of Chemistry at the University of Calgary. Research Abstract “Equilibrium Water Contents for Acid Gas Mixtures Destined for Sequestration” – R. A. Marriott, E. Fitzpatrick, F. Bernard, H. H. Wan, K. L. Lesage, P. M. Davis & P. D. Clark Re-injection of acid gases (CO2 and H2S) enables energy companies to reduce greenhouse gas emissions and reduce environmental impact. Understanding the acid gas and water equilibria is an important aspect for designing safe and reliable injection facilities and selecting appropriate injection zones. Accurate prediction of water content is particularly important for multiple stage compression of high-CO2 and / or high-H2S process gases to dense phase injection fluids. For example, a free water phase can lead to corrosion issues within a wellbore or an under-saturated fluid could dehydrate the near wellbore regions of a target reservoir. In collaboration with the Department of Chemical Engineering, in 2001 ASRL began a joint industry project to measure water contents for acid gas mixtures under moderate pressures and temperatures (T = 25 to 60˚ C and p = 10 to 150 bar). To date, ASRL has reported over 160 data points, which is more than half of those available in the literature. The scarcity of data is due, in large part, to the experimental difficulty in obtaining representative samples of equilibrated dense phase acid gases (liquids and supercritical fluids). Over the last eight years, ASRL has used several techniques to obtain these measurements, but recently they have all evolved into two primary techniques: 1) visual dew point determination for liquid acid gas + hydrates, and 2) an isolated floating piston with a microsampler for near-direct GC injection of gaseous or liquid acid gas phases. As the Joint Industry Project was coming to an end, the microsampler technique also evolved in capability. The high-pressure limit is now 1000 bar, which includes nearly any injection reservoir pressure. Note that 0 – 100% H2S can be accommodated. Among the current ASRL projects is the investigation of pure H2S + water equilibria at very high pressures and the testing of a new Thermionic GC detector (TID-1) which is tuned for water. In addition, the Gas Processor’s Association has recently shown interest in utilizing ASRL’s capabilities to revisit the water content data available in the GPA Handbook. These techniques and some recent data have been summarized within the research poster.