The application of proteomic discovery and development to clinical
advertisement

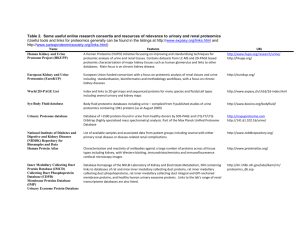
1 Table 1: Studies selected for review according to search criteria (those marked * discussed in more detail in the main text) Renal condition Acute Kidney Injury (AKI) Samples† Urine, rat (sepsis model, n=3) Urine, rat (ischaemia reperfusion model, n=3) Urine, rat (cisplatin induced AKI, ischaemia reperfusion, volume depletion; n=12 per group, pooled) Urine, rat (4-aminophenol model, n=5 for 3 dosage levels) Urine (CPB, n=22) Urine (Intensive care patients post CABG, n = 20) Urine (Contrast nephropathy, n=10) Rat proximal tubular cell lysates exposed DCVC Lysed rat IMCD cell isolates from lithium treated rats Rat renal cortex +/- gentamicin exposure (n=5 for 3 dosage groups) Kidney sections from gentamicin treated rats (n=3) Mouse model of aristolochic acid nephropathy (n=12 total) Lead toxicity in rats, medulla + cortex rat kidney homogenates (n=5) Urine, rats undergoing total body irradiation Urine (Paediatric patients undergoing cardiac bypass, n=32) Urine (n=22) Biomarkers detected/identified and other findings (proteomic technique employed‡) Mephrin-1α-decrease , reduction in serine-protease inhibitors and uromodulin (2D-DIGE) Sex-specific meprin-α increase in males (2D-DIGE) Increased exosomal Fetuin-A which preceeded changes in creatinine in AKI from cisplatin and ischaemia reperfusion injury, but not in volume depletion (2D-DIGE) Increased fumarylacetoacetate (before creatinine) /T-kininogen/inter-alpha-inhibitor H4P heavy chain (2D-DIGE) Hepcidin-25 less commonly observed in AKI-patients than controls (SELDI-TOF-MS) Increased α-1-microglobulin and β-2-microglobulin in AKI (SELDI-TOF-MS and 2D-DIGE) Variant of human β-defensin-1 elevated in patients less likely to develop nephropathy (SELDI-TOF-MS). Increased phosphorylation of actin-related protein-2 (2D-PAGE) Several signalling pathways altered including PkB/Akt, p38 and JNK. ↓aquaporin expression with Li+ (2D-DIGE) 20 proteins altered (e.g. carbohydrate metabolism, fatty acid oxidation, oxidiative phosphorylation) (2D-PAGE) Transthyretin-fragment differentiated normal kidneys from gentamicin nephrotoxicity (MALDI-Imaging) Mouse urinary protein↓; serum amyloid, transthyretin, and others all ↑ (1D-PAGE and LC-MS/MS) 76 cortical proteins altered, though only 13 medullary proteins altered; e.g. aldose reductase↑ (medulla), α 2 microglobulin ↓(medulla and cortex), transferrin ↓(medulla and cortex) - (2D-PAGE) 264 proteins altered after irradiation, significant changes in 24h (2D-PAGE and LC-MS/MS) In those given it, urinary aprontinin levels ↑ 2h after CPB in those who went on to develop AKI (SELDI-TOF-MS) Diabetic 11 differentially expressed proteins identified distinguishing DN samples and normal (including transthyretin Nephropathy (DN) precursor, IgG-chains and retinol-binding protein) (2D-PAGE) Urine (n=89) 65 peptides detected which differentiate diabetics from normal; fragments of collagen I accounted for a number of the peaks seen (CE-MS) Urine (n = 112) 2000 peptides detected which differentiate diabetics from normal (CE-MS) Urine (n=58) 11 of 113 polypeptides that appeared to represent renal damage identified. 15 of 113 changed in the macroalbuminuric subgroup with candesartan treatment (CE-MS) Urine (n=80, total) Changes in collagen α-1 and α-5 fragments seen in DN (MALDI-TOF-MS) Urine (n = 21) Peptide fragments of urinary collagen decreased in progressors, over 10-12 years (MALDI-TOF-MS) Urine (n=32, total) Soluble E-cadherin upregulated with increasing proteinuria (2D-DIGE) Urine (n = 3) Alpha-1-antitrypsin increase, localised to areas of renal fibrosis by subsequent immunostaining (2D-DIGE) OVE26 mouse model - whole kidney extract (n=5) 92 proteins identified as altering in diabetes including ↑ renal elastase inhibitor and ↓ elastase IIIB (2D-PAGE) Urine, rat (streptozotozin model) 19 altered proteins, inc. ↑pro-α-collagen, confirmed with selected reaction monitoring (Label-free LC-MS/MS) Lysates of skin fibroblasts from diabetics (n=15, total) Altered cytoskeletal proteins (including α-actinin-4 and moesin) in DN-patient fibroblasts (2D-PAGE) Glomerular Plasma (n=34) Oxidised forms of albumin detected in patients with FSGS compared to other diseases and normals (LC-MS/MS) diseases Urine (n=17) Fragments of albumin and α1-antitrypsin present representing 72 altered protein spots (2D-PAGE) 1)Nephrosis Urine (paediatric, SSNS n=14, SRNS n=11) Unidentified 4144kDa peak discriminated SSNS vs SRNS, though PCRs varied significantly (SELDI-TOF-MS) Glomeruli from 5/6 nephrectomised rats (n=3) ↑thymosin-β4 in sclerotic glomeruli, possibly related to inhibition of ECM degradation (MALDI-TOF-MS) Glomeruli from 5/6 nephrectomised rats (n=6) 33 proteins altered inc. dimethylarginine dimethylaminohydrolase-1, possible link to ↑vascular tone (2D-DIGE) Cultured murine podocytes 106 proteins differ in podocytes upon exposure to dexamethasone including cytoskeletal proteins, ciliary neurotrophic factor, αB-crystallin, and 3 proteins with cellular protective roles (2D-PAGE) †Samples are human unless specified; n numbers refer to number with condition unless otherwise stated; where >2 groups were used in a study, a total n number is usually given ‡Abrreviations used for proteomic techniques defined both in text and Boxes. Other abbreviations: CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; DCVC, dichlorovinyl cysteine; ECM, extra-cellular matrix; FSGS, focal segmental glomerulosclerosis; IMCD, inner medullary collecting duct; PCR, protein creatinine ratio; SSNS, steroid sensitive nephrotic syndrome; SRNS, steroid resistant nephrotic syndrome Ref. 1 2 3* 4* 5* 6 7* 8 9* 10* 11 12 13 14 15 16* 17* 18* 19* 20* 21* 22* 23* 24* 25* 26 27* 28* 29* 30* 31* 32 2 Table 1: Studies selected for review according to search criteria continued (those marked * discussed in more detail in the main text) Renal condition Glomerular diseases 2)IgA Nephropathy 3)Lupus Nephritis (LN) 3)Membranous Nephropathy (MN) 4)AAV Kidney Stone Disease Haemodialysis and haemofiltration Samples† Urine (n=45) Urine (n=17) Urine (n=18) Urine (n = 19; 25 longitudinal samples during 25 flare cycles) Urine (n=55) Urine, rat model of MN (n=10) Urine, rat model of MN (n=6) Urine (n=37) MDCK cells exposed to calcium oxalate monohydrate (n=5) MDCK cells exposed to calcium oxalate dihydrate extract (n=5) Stone Matrix Proteins, 3 stones from 3 different individuals Haemodialysis patients (n=4) Haemodialysis patients divided by low vs high flux filter types (n = 26), peritoneal dialysis patients (n=10) Slow Continuous Ultrafiltrate (1 patient with AKI) Biomarkers detected/identified and other findings (proteomic technique employed‡) Peptide patterns distinguished IgA nephropathy from controls and other nephropathies (CE-MS) Ratio of α1-microglobulin:(albumin/transferrin) distinguished IgA nephropathy from controls and DN (2D-PAGE) ↑kininogen and others, and ↓transthyretin in ACE-inhibitor responders cf. non-responders (2D-PAGE) Increased hepcidin-20 four months pre-flare; decreased hepcidin-25 during renal flare (SELDI-TOF-MS) Peaks m/z 3340 and 3980Da distinguished active vs inactive LN (SELDI-TOF-MS) Prehaptoglobin and haptoglobin increased in urine from compared to controls (2D-DIGE) 37 differentially expressed proteins involved in glomerular trafficking/permeability (2D-PAGE) 113 polypeptides distinguish AAV from controls (58 sequenced, mostly haemoglobin fragments) (CE-MS) 53 proteins altered and interaction with calcium oxalate monohydrate promoted cell death (2D-PAGE) 11 proteins altered, including annexin 2, which is known to bind calcium oxalate (2D-PAGE) 158 proteins seen inc. calcium binding and inflammatory proteins; 28 seen in all 3 stones (1D-PAGE, LC-MS/MS) 30 peaks detected as differing-peptides responsible not identified (SELDI-TOF-MS) Distinct protein profiles between groups-high flux dialysates contained β2-micoglobulin, α-defensins, and inflammatory proteins. Low flux filters released virtually no mid- to high-MW proteins (SELDI-TOF-MS) Potentially beneficial losses, e.g. zinc α2-glycoprotein; potentially detrimental losses, e.g. albumin (gel-LCMSMS) 292 proteins seen inc. N-Ac proteins and proteins < 40kDa, some not known in serum (1D-PAGE, LC-MSMS) 58 proteins altered on exposure to PD fluid; possible adaptive response on repeated exposure (2D-PAGE) C4A, Ig κ light chain VLJ region, albumin, α1-antitrypsin, apo-AI altered in varying membrane types (2D-PAGE) Cleaved β2-microglobulin increase seen in AR not other groups, including acute tubular necrosis (SELDI-TOF-MS) Cleaved β2-microglobulin peak, NGAL, α1-m, RBP all altered but none statistically significant (SELDI-TOF-MS) Peaks representing α-1-antichymotrypsin and β-defensin-1 discriminated AR (SELDI-TOF-MS) Urinary polypeptide pattern identified 16 of 19 ARs, and 6 of 9 in validation set (CE-MS) Increases in several proteins seen in chronic allograft nepropathy e.g. α1-m, β2-m, endorepellin (SELDI-TOF-MS) Peak cluster m/z 1539.8-1657.4Da differentiated IF/TA from CAAR (MALDI-TOF-MS) Uromodulin and kininogen derived peaks m/z 645.9 and 642.61Da differentiated CAD groups (Label-free MS) 6 peaks identified which distinguished BK nephropathy from normal urine and acute rejection (SELDI-TOF-MS) 197 proteins differed between normal and PKD. 38sequenced - mainly collagen fragments (CE-MS) Alteration of proteins involved e.g. in cell-proliferation, cell-matrix contact, fluid secretion (iTRAQ, LC-MS/MS) Increased expression of numerous proteins linked to polycystin / fibrocystin signalling pathway (2D-PAGE) 552 differential proteins including polycystin-1 and -2, cystin and ADP-ribosylation factor like-6 (2D-PAGE) Peptides unique to Dent’s seen e.g. leukotactin-I, IGF-I / II and growth factors (1D + 2D-PAGE , LC-MS/MS) 30 proteins enabled discrimination of Dent’s / Lowe’s vs. controls (1D and 2D-PAGE, LC-MS/MS) Increased phosphorylation of AQP2, AQP4 and urea-transport isoforms-A1 and A3 (LC-MS/MS) Phosphorylated AQP2 enriched in DRM. Vasopressin treatment caused ↓Rab7, annexin-2 in DRM (LC-MS/MS) ↑phosphorlyation of myosin-light chain IIA/B via MLCK. MLCK-inhibitors ↓IMCD water permeability (2D-PAGE) Pathway analysis revealed 33 proteins involved in vasopressin-escape (2D-DIGE) Ref. 33* 34 35 36* 37* 38 39 40* 41 42 43 44* 45* 46 CVVH effulent (1 patient with AKI following CPB) 47* Mesothelial cell lysate (n=16 for control and treated cells) 48 Peritoneal dialysate fluid (n=20, total) 49* Urine (n=83, total) 50* Urine, trying to detect subclinical tubultits (n=100, total) 51* Urine (n=65, total) 52* Urine (n=58, total) 53* 2)Chronic Allograft Urine (n=75, total) 54* Dysfunction (CAD) Urine (n=50, total) 55* Urine (n=32; separate validation set also included) 56* 3) BK nephropathy Urine (n=21) 57 Polycystic Kidney Urine (n = 17, separate validation set also included) 58* Disease (PKD) Membrane fractions from PDK1-/- mouse models 59* Kidney homogenate from jck-mutant mice (n=3) 60* Urinary exosome fraction from PKHD1-/- mice 61* Fanconi Syndrome Urine (n=4) 62* (FS) Urine (n=28) 63* Fluid and Rat IMCD cells exposed to vasopressin 64* electrolyte Profiling of rat CDC detergent-resistant membrane (DRM) 65* disorders Rat IMCD cells exposed to vasopressin +/- inhibitor 66* Vasopressin treated rat IMCD cells (n=4) +/- vasopressin 67* escape Rabbit LoH cells, low vs. high resistance to osmotic stress 40 proteins differentially expressed inc. sorbitol pathway enzymes, cytoskeletal proteins and HSPs (2D-PAGE) 68* IMCD cells adapted to hypertonicity vs controls Increased expression of S100A4 in both acute and chronic hypertonicity (2D-DIGE) 69* †Samples are human unless specified; n numbers refer to number with condition unless otherwise stated; where >2 groups were used in a study, a total n number is usually given ‡Abrreviations used for proteomic techniques are defined in text and Boxes. Other abbreviations: AAV, ANCA associated vasculitis; ACE, angiotensin converting enzyme; AQP, aquaporin; CAAR, chronic active antibody-mediated rejection; CVVH, continuous veno-venous haemofiltration; HSP, heat-shock protein; IF/TA, interstitial fibrosis/tubular atrophy; IMCD, inner medullary collecting duct; LoH, loop of Henle; N-Ac, N-acetylated; NGAL, neutrophil gelatinase associated lipocalin; MDCK, Madin-Darby canine kidney; MLCK, myosin light-chain kinase; RBP, retinol binding protein; α1-m, alpha 1 microglobulin; β2-m, beta 2 microglobulin Peritoneal Dialysis (PD) Transplantation 1)Acute Rejection (AR) 3 References for Table 1 1. Holly, M.K. et al. Biomarker and drug target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int. 70, 496-506 (2006). 2. Takayama, J. et al. Sex difference in ischemic acute renal failure in rats: approach by proteomic analysis. Biol. Pharm. Bull. 30, 1905-1912 (2007). 3. Zhou, H. et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 70, 1847-1857 (2006). 4. Bandara, L.R. et al. A potential biomarker of kidney damage identified by proteomics. Biomarkers 8, 272-286 (2003). 5. Ho, J. et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am. J. Kidney Dis. 53, 584-595 (2009). 6. Vanhoutte, K.J.A. et al. Biomarker discovery with SELDI-TOF-MS in human urine associated with early renal injury: evaluation with computational analytical tools. Nephrol. Dial. Transplant 22, 2932-2943 (2007). 7. Bennett, M.R. et al. Using proteomics to identify preprocedural risk factors for contrast induced nephropathy. Proteomics Clin. Appl. 2, 1058-1064 (2008). 8. de Graauw, M. et al. Proteomic analysis of alternative protein tyrosine phosphorylation in 1,2-dichlorovinyl-cysteine-induced cytotoxicity in primary cultured rat renal proximal tubular cells. J. Pharmacol. Exp. Ther. 322, 89-100 (2007). 9. Nielsen, J. et al. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc. Natl. Acad. Sci. U.S.A. 105, 3634-3639 (2008). 10. Charlwood, J. et al. Proteomic analysis of rat kidney cortex following treatment with gentamicin. J. Proteome Res. 1, 73-82 (2002). 11. Meistermann, H. et al. Biomarker discovery by imaging mass spectrometry. Transthyretin is a biomarker for gentamicin-induced nephrotoxicity in rat. Mol. Cell. Proteomics 5, 1876-1886 (2006). 12. Huang, F. et al. SELDI-TOF as a method for biomarker discovery in the urine of aristolochic-acid-treated mice. Electrophoresis 30, 1168-1174 (2009). 13. Witzmann, F.A. et al. Regional protein alterations in rat kidneys induced by lead exposure. Electrophoresis 20, 943-951 (1999). 14. Sharma, M. et al. The urine proteome as a biomarker of radiation injury. Proteomics Clin. Appl. 2, 1065-1086 (2008). 15. Nguyen, M.T. et al. Urinary aprotinin as a predictor of acute kidney injury after cardiac surgery in children receiving aprotinin. Pediatr. Nephrol. 23, 1317-1326 (2008). 4 16. Bellei, E. et al. Proteomic analysis of early urinary biomarkers of renal changes in type 2 diabetic pateients. Proteomics Clin. Appl. 2, 478-491 (2008). 17. Rossing, K. et al. Urinary proteomics in diabetes and CKD. J. Am. Soc. Nephrol. 19, 1283-1290 (2008). 18. Mischak, H. et al. Proteomic analysis for the assessment of diabetic renal damage in humans. Clin. Sci. 107, 485-495 (2004). 19. Rossing, K. et al. Impact of diabetic nephropathy and angiotensin II receptor blockade on urinary polypeptide patterns. Kidney Int.. 68, 193-205 (2005). 20. Lapolla, A. et al. Low molecular weight proteins in urines from healthy subjects as well as diabetic, nephropathic and diabetic-nephropathic patients: a MALDI study. J. Mass Spectrom. 44, 419-425 (2009). 21. Merchant, M.L. et al. Urinary peptidome may predict renal function decline in type 1 diabetes and microalbuminuria. J. Am. Soc. Nephrol. 20, 2065-2074 (2009). 22. Jiang, H. et al. Identification of urinary soluable E-cadherin as a novel biomarker for diabetic nephropathy. Diab. Metab. Res. Rev. 25, 232-241 (2009). 23. Sharma, K. et al. Two-dimensional fluorescence difference gel electrophoresis analysis of the urine proteome in human diabetic nephropathy. Proteomics 5, 2648-2655 (2005). 24. Thongboonkerd, V. et al. Alterations in the renal elastin-elastase system in type I diabetic nephropathy identified by proteomic analysis. J. Am. Soc. Nephrol. 15, 650-662 (2004). 25. Schlatzer, D.M. et al. Urinary protein profiles in a rat model for diabetic complications. Mol. Cell. Proteomics 8, 2145-2158 (2009). 26. Millioni, R. et al. Abnormal cytoskeletal protein expression in cultured skin fibroblasts from type I diabetes mellitus patients with nephropathy: a proteomic approach. Proteomics Clin. Appl. 2, 492-503 (2008). 27. Musante, L., et al. Active focal segmental glomerulosclerosis is associated with massive oxidation of plasma albumin. J. Am. Soc. Nephrol. 18, 799-810 (2007). 28. Candiano, G. et al. Repetative fragmentation products of albumin and α1-antitrypsin in glomerular diseases associated with nephrotic syndrome. J. Am. Soc. Nephrol. 17, 3139-3148 (2006). 29. Woroniecki, R.P. et al Urinary proteome of steroid sensitive and steroid resistant idiopathic nephrotic syndrome of childhood. Am. J. Nephrol. 26, 258-267 (2006). 30. Xu, B.J. et al. Proteomic patterns and prediction of glomerulosclerosis and its mechanisms. J. Am. Soc. Nephrol. 16, 2967-2975 (2005). 31. Potthoff, S.A. et al. The glomerular proteome in a model of chronic kidney disease. Proteomics Clin. Appl. 2,1127-1139 (2008). 5 32. Ransom, R.F., Vega-Warner, V., Smoyer, W.E. & Klein, J. Differential proteomic analysis of proteins induced by glucocorticoids in cultured murine podocytes. Kidney Int. 67, 1275-1285 (2005). 33. Haubitz, M. et al. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int. 67, 2313-2320 (2005). 34. Yokota, H. et al. Absence of increased α1-microglobulin in IgA nephropathy proteinuria. Mol. Cell. Proteomics 6, 738-744 (2007). 35. Rocchetti, M.T. et al. Urine protein profile of IgA nephropathy patients may predict response to ACE-inhibitor therapy. Proteomics 8, 206-216 (2008). 36. Zhang, X. et al. Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int.. 74, 799-807 (2008). 37. Mosley, K. et al. Urinary proteomic profiles distinguish between active and inactive lupus nephritis. Rheumatology 45, 1497-1504 (2006). 38. Ngai, H.H., Sit, W.H., Jiang, P.P., Thongboonkerd, V. & Wan, J.M. Markedly increased urinary preprhaptoglobin and haptoglobin in passive Heymann nephritis: a differential proteomics approach. J. Proteome Res. 6, 3313-3320 (2007). 39. Ngai, H.H. et al. Serial changes in urinary proteome profile of membranous nephropathy: implications for pathophysiology and biomarker discovery. J. Proteome Res. 5, 3038-3047 (2006). 40. O’Riordan, E. et al. Bioinformatic analysis of the urine proteome of acute allograft rejection. J. Am. Soc. Nephrol. 15, 3240-3248 (2004). 41. Thongboonkerd, V., Semangoen, T., Sinchaikul, S. & Chen, S. Proteomic analysis of calcium oxalate monohydrate crystal-induced cytotoxicity in distal renal tubular cells. J. Proteome Res. 7, 4689-4700 (2008). 42. Semangoen, T., Sinchaikul, S., Chen, S. & Thongboonkerd, V. Altered protein in MDCK renal tubular cells in response to calcium oxalate dehydrate crystal adhesion: A proteomics approach. J. Proteome Res. 7, 2889-2896 (2008). 43. Merchant, M.L. et al. Proteomic analysis of renal calculi indicates an important role for inflammatory processes in calcium stone formation. Am. J. Physiol. Renal Physiol. 295, F1254-F1258 (2008). 44. Langlois , R.G. et al. Serum protein profile alterations in hemodialysis patients. Am. J. Nephrol. 24, 268-274 (2004). 45. Dihazi, H., Muller, C.A., Mattes, H. & Muller, G.A. Proteomic analysis to improve adequacy of hemo- and peritoneal dialysis: Removal of small and high molecular weight proteins with high- and low-flux filters or a peritoneal membrane. Proteomics Clin. Appl. 2, 1167-1182 (2008). 6 46. Lefler, D.M., Pafford, R.G., Black, N.A., Raymond, J.R. & Arthur, J.M. Identification of proteins in slow continuous ultrafiltrate by reversed-phase chromatography and proteomics. J. Proteome Res. 3, 1254-1260 (2004). 47. Molina, H. et al. A proteomic analysis of human hemodialysis fluid. Mol. Cell. Proteomics 4, 637-650 (2005). 48. Kratochwill, K. et al. Stress responses and conditioning effects in mesothelial cells exposed to peritoneal dialysis fluid. J. Proteome Res. 8, 1731-1747 (2009). 49. Sritippayawan, S. et al. Proteomic analysis of peritoneal dialysate fluid in patients with different types of peritoneal membranes. J. Proteome Res. 6, 4356-4362 (2007). 50. Schaub, S. et al. Proteomic-based identification of cleaved urinary β2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am. J. Transplant. 5, 729-738 (2005). 51. Schaub, S. et al. Detection of subclinical tubular injury after renal transplantation: Comparison of urine protein analysis with allograft histology. Transplantation 84, 104112 (2007). 52. O’Riordan, E. et al. Characterisation of urinary peptide biomarkers of acute rejection in renal allografts. Am. J. Transplant. 7,930-940 (2007). 53. Wittke, S. et al. Detection of acute tubulointerstitial rejection by proteomic analysis of urinary samples in renal transplant recipients. Am. J. Transplant. 5, 2479-2488 (2005). 54. O’Riordan, E. et al. Urinary proteomic analysis of chronic allograft nephropathy. Proteomics Clin. Appl. 2, 1025-1035 (2008). 55. Quintana, L.F. et al. Urine proteomics to detect biomarkers for chronic allograft dysfunction. J. Am. Soc. Nephrol. 20, 428-435 (2009). 56. Quintana, L.F. et al. Application of label-free quantitative peptidomics for the identification of urinary biomarkers of kidney chronic allograft dysfunction. Mol. Cell. Proteomics 8, 1658-1673 (2009). 57. Jahnukainen, T. et al. Proteomic analysis of urine in kidney transplant patients with BK nephropathy. J. Am. Soc. Nephrol. 17, 3248-3256 (2006). 58. Kistler, A.D. et al. Identification of a unique urinary biomarker profile in patients with autosomal dominant polycystic kidney disease. Kidney Int. 76, 89-96 (2009). 59. Han, C. et al. A multiplexed quantitative strategy for membrane proteomics: opportunities for mining therapeutic targets for autosomal dominant polycystic kidney disease. Mol. Cell. Proteomics 7, 1983-1997 (2008). 60. Valkova, N., Yunis, R. Mak. S.K., Kang, K. & Kultz, D. Nek8 mutation causes overexpression of galectin-1 sorcin, and vimentin and accumulation of the major urinary protein in renal cycst of jck mice. Mol. Cell. Proteomics 4, 1009-1018 (2005). 7 61. Hogan, M.C. et al. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 20, 278-288 (2009). 62. Cutillas, P.R. et al. The urinary proteome in Fanconi syndrome implies specificity in the reabsorption of proteins by renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 287, F353-64 (2004). 63. Vilasi, A. et al. Combined proteomic and metabonomic studies in three genetic forms of the renal Fanconi syndrome. Am. J. Physiol. Renal Physiol. 293, F456-F467 (2007). 64. Hoffert, J.D., Pisitkun, T., Wang, G., Shen, R. & Knepper, M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc. Natl. Acad. Sci. U.S.A. 103, 7159-7164 (2006). 65. Yu, M.J. et al. Large scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am. J. Physiol. Cell Physiol. 295, C661-C678 (2008). 66. Chou, C. et al. Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signalling in the renal collecting duct. J. Biol. Chem. 279, 4902649035 (2004). 67. Hoorn, E.J., Hoffert, J.D. & Knepper, M.A. Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J. Am. Soc. Nephrol. 16, 2852-2863 (2005). 68. Dihazi, H., Asif, A.R., Agarwal, N.K., Doncheva, Y. & Muller, G.A. Proteomic analysis of cellular response to osmotic stress in thick ascending limb of Henle’s loop (TALH) cells. Mol. Cell. Proteomics 4, 1445-1458 (2005). 69. Rivard, C.J. et al. Expression of the calcium-binding protein S100A4 is markedly upregulated by osmotic stress and is involved in the renal osmoadaptive response. J. Biol. Chem. 282, 6644-6652 (2007).