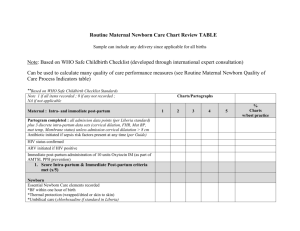
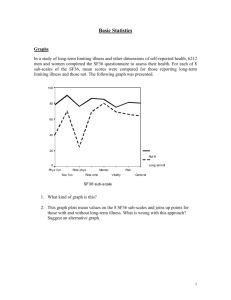
View/Open - Minerva Access
advertisement

Chapter 5 Maturation of the growth axis in the developing tammar wallaby Chapter 5 Maturation of the growth axis in the developing tammar wallaby INTRODUCTION The GH-IGF-1 axis controls somatic growth in most vertebrates (Laron et al., 1966; Liu et al., 1993; Gluckman and Pinal, 2003). In mammals, IGF-1 and 2 concentrations and the rate of growth before birth depend on the level of nutrition, provided by the placenta (Gluckman et al., 1987; Bauer et al., 1995; Oldham et al., 1999). However, the hypothalamo-pituitary growth axis matures during the perinatal period, characterised by upregulation of hepatic growth hormone receptors (GH-Rs) and the high affinity GH-binding protein (GHBP) (Gluckman et al., 1983; Maes et al., 1983; Badinga et al., 1991; Schnoebelen-Combes et al., 1996). One hypothesis to explain the dramatic shift in the control of growth at birth is that regulation of fetal IGF-1 allows growth to be restrained relative to the nutritional capabilities of the mother and placenta, preventing fetal overgrowth and possible pregnancy complications (Gluckman and Pinal, 2003). The binding of GH to its receptors in the liver directly stimulates hepatic IGF-1 production which in turn provides an inhibitory signal to the brain to reduce GH synthesis (Gluckman et al., 1979a; Berlowitz et al., 1981; Bichell et al., 1992). GH also stimulates the production of other growth axis genes including IGF-binding protein-3 (IGFBP-3) and the acid labile subunit (ALS) that forms a ternary complex with IGFs and IGFBP-3 to assist in their preservation and transport in the blood (Scott and Baxter, 1991; Domene et al., 1993; Baumann et al., 1994; Ooi et al., 1997. This provides the neonatal young with autonomy to control its own somatic growth via GH production. Similar molecular mechanisms leading to growth independence of the offspring occur in another small marsupial, the brush-tailed possum, Trichosurus vulpecula (Saunders, et al., 2003). However, these changes took place during the extensive period of lactation whilst the young was in the pouch, so the maturation of GHregulated growth developed around the time of first pouch exit (Saunders et al 2003), a period equivalent to birth in precocial eutherian species (Renfree, 1993). Thus somatotrophic control appears to develop at a similar pace in all mammals, regardless of whether it occurs peri-natally, as in eutherians, or much later relative to the time of birth, as in marsupials. 89 Chapter 5 Maturation of the growth axis in the developing tammar wallaby In addition to the age related changes in growth axis genes that have been described in the brush-tailed possum and many eutherian species, there are also sexual dimorphisms in the growth axis. Androgens produced by the male fetus are thought to imprint later GH pulsatility accounting for the differences in body size between males and females (Jansson et al., 1984). There are sexually dimorphic plasma concentrations of IGF-1, IGF-2 and IGFBP-3 in growing lambs that may also account for growth differences (Gatford et al., 1996). In the tammar, testicular androgens secreted between day 2 and day 50 (Renfree et al., 1992) imprint later growth and development of the male phallus and are essential for urethral closure (Leihy et al., 2004). However, after the phallus becomes sexually dimorphic between days 70 and 100, other factors besides androgen must contribute to the continued penile growth because there is no difference in the plasma androgens of males and females during this period (Wilson et al, 2003; Leihy et al., 2004; Shaw et al, 2000). Marsupials deliver highly altricial young, so growth and development is not constrained by the uterus because it occurs post-natally, usually within a pouch. The tammar wallaby neonate weighs 400mg at birth, only 0.1% of maternal weight, and subsequent growth of their organ systems occurs over an extended period whilst they are in the pouch (Tyndale-Biscoe and Renfree, 1987). In particular, the gonads are undifferentiated at birth (O et al., 1988) and sexual differentiation of the reproductive system occurs during the first 150 days post-natally (Renfree et al., 1996). Thus the marsupial young is essentially equivalent to an exteriorised fetus that is accessible for endocrine or nutritional manipulation. This chapter therefore investigates the expression profile of key growth genes, namely hepatic IGF-1 and 2, GHR, and IGFBP-3, as well as the profile of plasma IGF-1 during the lengthy period of postnatal development of male and female tammar wallabies. 90 Chapter 5 Maturation of the growth axis in the developing tammar wallaby METHODS Species specific primers for GH, IGF-1, IGF-2, GH-R and IGFBP-3 were designed from the full length sequences described in chapter 4 for use in qPCR (Table 12). Table 12. Oligonucleotide primers used for qPCR analysis of GH, GH-R, IGF-1, IGF-2 and IGFBP-3 expression and β-actin control sequence. Primer Name GH Sequence 5’–3’ GH-RT-F GH-RT-R GGACCTGGAAGAAGGGATTC CACAGCTGCTTTCCACAAAA GH-R Sequence 5’-3’ GHR-RT-F GHR-RT-R ACCTCCCAATGCAGATGTTC ATCCGAGGTACGTTGTCTGG IGF-1 Sequence 5’-3’ IGF1-RT-F IGF1-RT-R GCAATTCTCTAAGTGCTGCC AGCATTCATCCACGATCCC IGF-2 Sequence 5’-3’ IGF2-RT-F IGF2-RT-R CCTTTGTGGTGGGAACTGGT GGATGGGGTCTTCGCTGGGCA IGFBP-3 Sequence 5’-3’ BP3-RT-F BP3-RT-R AGACCCTTACAGGCGCTCTT CTTTGGCTTGTCCTTTCCTG β-actin Sequence 5’-3’ bACT-RT-F bACT-RT-R TTGCTGACAGGATGCAGAAG AAAGCCATGCCAATCTCATC The quantities of transcripts from the genes of interest were compared to the housekeeping gene β-actin (provided by Dr. Eleanor Ager). -actin was used because its expression has been shown not to differ significantly over the course of development in the possum (Saunders et al., 2001). Pituitary GH expression data were log transformed while the IGF-1 and GH-R expression data are plotted on a log scale. All other gene and protein expression measurements are plotted on a linear scale. 91 Chapter 5 Maturation of the growth axis in the developing tammar wallaby Total plasma IGF-1 concentrations were measured using a human IGF-1 kit (Bioclone, Sydney). This kit has been previously validated in our lab for measuring IGF-1 in milk. The sensitivity of the assay as indicated by the manufacturer was 1 ng/mL. All samples were run at the same time using reagents from the same kit including 9 quality control samples producing an intra-assay coefficient of variation of 12%. Statistical analyses were carried out as described in chapter 2. To validate that there was parallelism in the assay a quality control sample was diluted 1:1, 1:2, 1:4 and 1:10. The IGF-1 concentrations of these dilutions were 283.9, 145, 40, and 0 ng/mL respectively. RESULTS Quantitative analysis of pituitary GH and hepatic GH-R expression during development in the tammar wallaby Pituitary GH expression declined significantly with age (ANOVA p<0.01; Figure 27) similar to the relationship described for plasma GH concentrations during fetal development in eutherian species and post-natal life in other marsupials (Bassett, Thorburn and Wallace, 1970; Kaplan, Grumbach and Shepard, 1972; Gluckman et al., 1979; Saunders et al., 2000; Saunders et al., 2002). GH expression dropped transiently at day 40 post-partum after which, expression increased until after day 120 post-partem when expression dropped further with the lowest levels of expression observed in the adult pituitary. Hepatic expression of GH-R increased significantly with age (ANOVA p<0.001; Figure 27) reaching a plateau at day 150 post-partum when post-hoc analysis identified significant increases compared to earlier ages. There was no effect of gender on the expression of GH-R (ANOVA p>0.05), therefore data from males and females was pooled. 92 Chapter 5 Maturation of the growth axis in the developing tammar wallaby Figure 27. Expression profile of pituitary GH and hepatic GH-R during development in the tammar wallaby. GH expression decreased significantly with age in the tammar (ANOVA p<0.01) similar to the changes observed in plasma GH concentrations during fetal development in eutherian mammals. The early decrease in pituitary GH expression observed at 40 days post-partum (indicated by the letter b) may indicate that regulatory processes in the wallaby are beginning to mature and to control the production of GH. There is also a significant decline between 120 and 180 days post-partum (p<0.01). (N = 3-4 per stage of mixed sex). Hepatic GH-R expression increased significantly with age (ANOVA p<0.001; N = 4-6 per stage). Expression at day 150 post-partum and adult were significantly higher than earlier stages (p<0.001) as determined by post-hoc analysis. Letters indicate significance relative to individual stages. Quantitative analysis of hepatic IGF-1 expression and plasma IGF-1 concentrations in the developing tammar wallaby Hepatic expression of IGF-1 increased significantly with age similar to GH-R (ANOVA p<0.005; Figure 28) reaching a plateau at 150 days post-partum. There was no effect of gender on the expression of IGF-1 (ANOVA p>0.05), therefore data from males and females was pooled. Pairwise comparisons between ages showed significant increases at age 150 post-partum and adults compared to all other time points (p<0.05). 93 Chapter 5 Maturation of the growth axis in the developing tammar wallaby Plasma concentrations of IGF-1 followed a similar trend to hepatic mRNA expression increasing significantly with age (ANOVA p<0.001; Figure 28) with maximum concentrations detected between day 180 and 250 post-partum. There was no effect of gender in plasma IGF-1 concentrations so individual male and female values were pooled per stage. IGF-1 increased from 280 ± 56 ng/mL at 20 days to 1051 ± 81 ng/mL at 250 days post-partum. IGF-1 concentrations then declined to adult levels plasma IGF-1 (ug/mL) hepatic IGF-1 expression relative to -actin (364 ± 33 ng/mL). Figure 28. Expression profile of hepatic IGF-1 and total plasma IGF-1 concentrations during development in the tammar wallaby. IGF-1 expression increased significantly with age (ANOVA p<0.005; N = 7-10 per stage) reaching a plateau at approximately 150 days of post-natal life. Post-hoc analysis showed significant increases at day 150 post-partum and adult compared to all other time points. Total plasma IGF-1 concentrations increased significantly with age (ANOVA p<0.001) similar to hepatic IGF-1 expression but peaking at day 250 post-partum (N = 6 per stage). Letters indicate significance relative to individual stages determined by post-hoc analysis. 94 Chapter 5 Maturation of the growth axis in the developing tammar wallaby Figure 29. Expression profile of hepatic IGF-2 and IGFBP-3 during development in the tammar wallaby. Hepatic IGF-2 and IGFBP-3 expression increased significantly with age (ANOVA p<0.001; N = 3-4 per stage) and was significantly elevated in males compared to females at day 70 and 70-100 post-partum respectively. Asterisks indicate significant differences (* = p<0.01, *** = p<0.001). Quantitative analysis of hepatic IGF-2 and IGFBP-3 expression in the developing tammar wallaby Hepatic IGF-2 and IGFBP-3 expression increased significantly with age (ANOVA p<0.001 respectively; Figure 29). However the expression of these genes was significantly different between sexes. IGFBP-3 expression was significantly higher in males compared to females (ANOVA p<0.001), and there were highly significant 95 Chapter 5 Maturation of the growth axis in the developing tammar wallaby differences at ages 70 and 100 post-partum (p<0.001) as determined by post-hoc analysis. IGF-2 was not significantly different overall based on gender (ANOVA p>0.05) but there was a significant sex*age interaction (ANOVA p<0.01). Post-hoc analysis showed significantly higher IGF-2 expression in males at day 70 post-partum than females (p<0.005). IGF-2 expression in males then fell dramatically after this time, and was significantly less (p<0.005) than females at day 150 post-partum. The differences in expression of IGF-2 and IGFBP-3 between males and females were not the result of weight differences between male and female pouch young, which did not differ significantly between 15 and 150 days post-partum (Figure 30). PY Weight (g) 1000 100 10 1 15 40 70 100 150 Age (days) post-partum Figure 30. Comparison of pouch young live weight prior to dissection. There was no difference in the weight of male and female pouch young from which liver samples were taken from day 15 to 150 post-partum (blue bars, males; pink bars, females; weight shown on a log scale; n=3-4 per stage). 96 Chapter 5 Maturation of the growth axis in the developing tammar wallaby DISCUSSION The growth axis in the tammar wallaby develops during post-natal life, over the course of its extended lactation period, as opposed to late fetal/early post-natal life as seen in eutherian mammals. Pituitary GH expression dropped significantly between 120 and 180 days post-partum while hepatic IGF-1 and GHR reached a plateau of expression over the same period indicative of developing central control of GH production. This relationship is highly conserved during vertebrate development with similar changes observed in humans, sheep rats and possibly chickens (Kaplan, Grumbach and Shepard, 1972; Gluckman et al., 1979; Glasscock et al., 1990; Tanaka et al., 1996). The importance of the early decline in GH expression at 40 days post-partum in the wallaby is unclear. A similar trend was observed between 12 and 25 days postpartum in the brushtail possum where pituitary GH (in ng/g body weight) declined significantly before increasing again (Saunders, Gemmell and Curlewis, 2003). This may be in response to negative feedback effects resulting from initial increases in hepatic IGF-1 and GH-R expression and plasma IGF-1 concentrations which begin to increase from day 40 post-partum in the wallaby. However, this would suggest that the regulatory mechanisms for controlling GH are in place by day 40. Alternatively, the neurons that secrete SRIH may be developing function at this time and may account for the variability in GH expression after 40 days. Exogenous SRIH administration in the fetal sheep can cause reductions in plasma GH from approximately 100 days of fetal life (Gluckman et al., 1979). Hepatic IGF-1 and GH-R expression and IGF-1 plasma concentrations increased gradually from day 40 to 150-180 post-partum after which expression reached a plateau. This parallel increase in IGF-1 and GH-R expression suggests that the process of GH binding to the GH-R directly stimulates increases in IGF-1 during development similar to that which occurs in eutherian mammals (Gluckman et al., 1983; Maes et al., 1983; Badinga et al., 1991; Saunders et al., 2003). IGF-1 concentrations in the adult were low similar to measurements made in adult eutherian mammals (Gluckman et al., 1979a). One possible explanation for the discrepancy between hepatic IGF-1 expression and plasma IGF-1 concentrations in the adult could 97 Chapter 5 Maturation of the growth axis in the developing tammar wallaby be that other tissues contribute to the IGF-1 pool prior to adulthood with this contribution declining after 250 days post-partum. Hepatic IGF-2 expression increased significantly over the course of pouch life suggesting that the liver is actually a major contributor to plasma IGF-2 levels during development. This provides evidence of an endocrine role for IGF-2 during development in addition to its known autocrine/paracrine actions. Hepatic IGFBP-3 expression followed a similar trend consistent with findings in eutherian species over a similar developmental period (Domene et al., 1993; Baumann, Shaw and Amburn, 1994). The sex-specific increases in IGFBP-3 and IGF-2 expression at day 70 and 100 post-partum do not coincide with sexually dimorphic body size or growth rate at this age. This may indicate that excess IGFBP-3 has bound excess IGF-2 to abolish any potential male growth discrepancies. Intriguingly, these increases do coincide with the start of sexually dimorphic phallus growth in males (Leihy et al., 2004), matching circumstantial clinical and experimental evidence in humans and other mammals suggesting a role for IGF in penile differentiation (Levy and Husmann, 1996; Laron and Klinger, 1998; Gatford et al., 1996; Liu et al., 2001). Thus, the phallus may be more responsive to IGF-2 during this period than other developing tissues. Furthermore it has been shown previously in the tammar that high levels of androgens during early development (20-40 days) imprint later sexually dimorphic phallus growth in males (Leihy et al., 2004). However, the mechanisms by which this occurs are unknown. At day 70 post-partum, hepatic IGF-2 expression in the male tammar reaches a peak in expression that is 8 times greater than adult levels and significantly higher than females at this age. If these differences in gene expression are representative of protein concentrations, then it is possible that circulating IGF-2 acting via the IGF-1R in the phallus may contribute to sexually dimorphic phallus growth. The changes in pituitary GH and hepatic IGF-1 and GH-R expression suggest that maturation of the endocrine growth axis is taking place between approximately 150 and 200 days post-partum in the tammar wallaby. This period of development is one of rapid increase in weight of the young and dramatic change in the quantity and constituents of the milk. At 250 days post-partum, the tammar weighs 10 times what it did at 150 days, while the relative levels of lipid and protein dramatically increase 98 Chapter 5 Maturation of the growth axis in the developing tammar wallaby in the milk and daily milk consumption doubles over the same period (Green et al., 1980; Green et al., 1988). Thyroid hormone also reaches peak concentrations in the tammar around 160 days post-partum and is thought to be involved in the development of endothermy which matures at approximately day 150 post-partum in synchrony with fur growth (Setchell, 1974). The cellular composition of the stomach also changes dramatically so that by day 170 post-partum there is a distinct forestomach and hindstomach morphology more typical of adult animals (Waite et al., 2005). This change must occur before the young begins to consume grass at approximately day 190-200 post-partum (Renfree, 1994). Thus, central control of growth develops at a time when the young is growing rapidly, thermoregulation is developing, and the transfer of resources from the mother is peaking similar to the late gestation sheep fetus. CONCLUSIONS The molecular mechanisms that lead to growth independence of mammalian young are highly conserved. Maturation of the growth axis as demonstrated by upregulation of hepatic IGF-1 and GH-R expression and IGF-1 plasma concentrations, and decline in pituitary GH expression occurs during the lengthy lactation period as opposed to late fetal/early neonatal life in precocial eutherian species such as the sheep and pig. In the tammar, these changes occur between 150 and 200 days post-partum which is just prior to first pouch exit, a period developmentally equivalent to birth in the sheep. 99