KS5 Extraction of Aluminium
advertisement

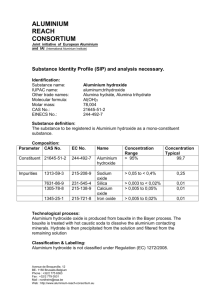
Extraction of Aluminium In groups arrange the following explanations in the correct order. The negative oxygen ions are attracted to the positive anode. The negative oxygen ions lose electrons and oxygen gas is given off. The following equation represents the reaction. 2 O2- 4eO2. In the ore there is a great deal of waste material including sand, iron (III) oxide. Before aluminium is extracted the ore has to be purified using sodium hydroxide. The product is pure alumina (Al2O3). Electrolysis is splitting compounds using electricity when the compound is in a molten (dissolved) state called an electrolyte. Bauxite is the ore, which is used for the extraction of aluminium. It is close to the surface and can be obtained by open-pit mining. The main problem with aluminium oxide it is an ionic compound with strong ionic bonds between the aluminium ions (Al3+) and the oxygen ions (O2-) so it has a very high melting point so the aluminium oxide needs a solvent to be dissolved in to. Aluminium is too high in the reactivity series to be obtained by reduction with carbon. Electrolysis has to be used for extraction on an industrial scale. The carbon electrodes, the anode (positive electrode) and the cathode (negative electrode), now separate the aluminium ions and oxygen ions. The positive aluminium ions are attracted to the negative cathode. The positive aluminium ions accept electrons and aluminium is deposited on the cathode. The following equation represents this Al3+ + 3eAl. The oxygen given off reacts with the anode carbon electrodes to form carbon dioxide. These anodes have to be replaced on a regular basis. Aluminium is tapped off from the electrolysis cell. Making 1 tonne of aluminium requires 5 tonnes of bauxite, 0.6 tonnes of carbon anodes, 0.45 tonnes of fuel oil, 0.08 tonnes of caustic soda and 0.05 tonnes of cryolite/fluorspar. The aluminium oxide is dissolved in to molten cryolite and fluorspar at 10000c . The aluminium ions and oxygen ions are now free to move in the solution.