C2-Chemistry-Questions
advertisement

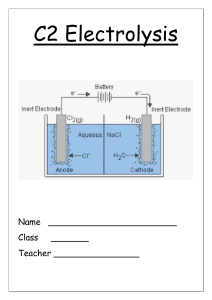
CHEMISTRY QUESTIONS 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) 13) 14) 15) 16) 17) 18) 19) 20) 21) 22) 23) 24) 25) 26) 27) 28) 29) 30) 31) 32) 33) 34) 35) 36) 37) 38) 39) 40) 41) 42) 43) What are thermosetting polymers? What are thermosoftening polymers? What sort of bonding is there in polymers? What is nanoscience? What are the uses of nanaoscience? Make a table of the advantages and disadvantages of nanoscience. What is the mass of a proton? What is the mass of a neutron? What is the mass of an electron? What are isotopes? How do you calculate teh number of neutrons in an atom? How do you work out the number of electrons? What element are all atoms compared to? What is the mass of 1 mole of carbon? How do you work out the percentage of an element in a compound? How do you calculate empirical formula? How do you calculate percentage yield? Why do we never get 100% yield? Draw the symbol for a reversible reaction. Why do we use chromatography? What do we use gas chromatography for? What does the mass spectrometer allow us to do? List how we can speed up the rate of reaction. How do we calculate the rate of reaction? How does a reaction happen? What does temperature do to the particles? How does surface area increase the rate of reaction? How does concentration increase the rate of reaction? If we increased the pressure of a gas what would that do to the particles? Why is a catalyst in the form of pellets more effective than a whole lump of catalyst? What are the pros and cons of using catalysts? What is an exothermic reaction? Explain in terms of bond making and bond breaking. What is an endothermic reaction? Explain in terms of bond making and bond breaking. Draw a graph to show endo and exothermic reactions. If a reaction is endothermic on the forward reaction what is it on the back reaction? What are the uses of exothermic reactions? What are the uses of endothermic reactions? What ions does an acid have? What ions does an alkali have? Describe how you could make a salt from an acid and alkali. Describe how you could make a salt from an acid and insoluble base. What type of reactions are these? What is a precipitate? 44) 45) 46) 47) 48) 49) 50) 51) 52) 53) 54) 55) 56) 57) 58) 59) 60) 61) 62) How do you make an insoluble salt? What is the positive electrode called? What is teh negative called? What are the ions called that are attracted to the cathode? What are the ions called that are attracted to the anode? Why does the electrolyte need to be molten or dissolved in water? What is oxidation? What is reduction? If an electrolyte is aqueous what ions can also be attracted to the electrodes? What are the uses of aluminium? What is the name of the aluminium ore? What is formed at each electrode in the electrolysis of aluminium oxide? Why is cryolyte used? Why does the anode need to be replaced regularly? Why is the extraction of aluminium expensive? Write half equations to show what happens at each electrode. What products do you get when you do the electrolysis of brine? List uses of chlorine, hydrogen, sodium hydroxide.