To set up the Ocean Optics Spectrophotometer, go to Start|Programs
advertisement

Determination of the Pseudo Rate Constant for the
Reduction Reaction of Methylene Blue
David Mork, Concordia College
INTRODUCTION:
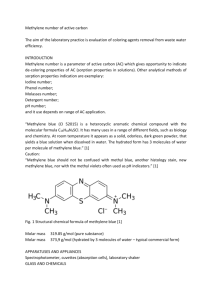
A schematic representation of the chemical reaction of methylene blue and ascorbic acid is
shown below. Methylene blue (MB+) is reduced to leucomethylene blue (LB+) by ascorbic acid
(H2A). Ascorbic acid is also called Vitamin C. Ascorbic acid is oxidized to dehydroascorbic acid
(D).
O
HO
N
O
+
(H3C)2N
S
+
H
CH2OH
HO
N(CH3)2
HO
methylene blue (MB+)
ascorbic acid (H2A)
H
O
N
O
(H3C)2N
S
+
+
N(HCH3)2
O
+
H
CH2OH
O
HO
leucomethylene blue (LB+)
dehydroascorbic acid (D)
We can symbolize this reaction as MB+ + H2A LB+ + D. The mechanism for this reaction has
never been fully investigated, but the kinetics of the reaction are easy to follow. The dark blue of
methylene blue disappears as the reaction progresses. The absorbance of light decreases as the
reaction goes to completion. You will record absorbance versus time using the Ocean Optics
spectrophotometer and computer program, and you will analyze your results using Excel.
You and your partner will be finding the pseudo rate constant,(k’), for methylene blue. The rate law
for this reaction has the form
Rate = k [MB+]m{H2A]n[HCl]p
The concentration of HCl and ascorbic acid are much higher than the concentration of methylene
blue. Over the length of our experiment, these two concentrations will not change and so we can
treat them as constants. The rate law becomes
Rate = k’ [MB+]m
where k’ = k[H2A]n[HCl]p.
Because we have not studied the kinetics of hydrochloric acid or ascorbic acid, we will not be able
to write a complete rate law for this reaction or determine the true rate constant, k.
EXPERIMENT:
You will follow the change in absorbance (which correlates to concentration) over time for two
different concentrations of methylene blue. If the spectrophotometer is busy, perform one
experiment and analyze your data in a computer lab.
1
2
0.10 M HCl
1.5 mL
1.5 mL
0.030 M Ascorbic Acid
1.0 mL
1.0 mL
1.0 x 10-4 M Methylene blue
0.50 mL
0.25 mL
You may not need to set up the computer program unless you are the first user. Regardless, you
should verify that the time acquisition settings are correct.
To set up the Ocean Optics Spectrophotometer, log into the computer using your username and
password. Go to Start|Programs|Ocean Optics|OOIBase 32|OOIBase 32. Turn on the lamp on the
spectrophotomer. The switch is on the blue part of the Ocean Optics equipment. You will see a
screen like this:
Integration
Time
The red intensity curve should NOT have a flat top. If it does, reduce the Integration Time until the
peak maximum is between 3000 and 4000.
The amount of noise in your spectrum can be reduced greatly by properly adjusting the average
and boxcar settings. Enter “20” for the average and “5” for the boxcar. These settings are found
just to the right of the Integration Time box.
After the lamp is warmed up, fill a cuvette with 1.5 mL of 0.1 M HCl and 1.0 mL of 0.030 M Ascorbic
acid. Wipe the outside of the cuvette with a Kimwipe and position the clear side of the cuvette in
the cuvette holder.
Store the reference spectrum by clicking the Store Reference button on the tool bar.
Store Dark
Store
Reference
Insert a piece of paper or your floppy disk in the opening in the blue part of the
spectrophotometer. Be sure to completely block the light source with the paper. Store the dark
spectrum by clicking the Store Dark button on the tool bar.
Now, configure the Ocean Optics program to record absorbance changes over time. Go to Time
Acquisition| Configure |Configure Acquisition. Set the boxes as they appear in the picture below.
Store you file under the A:\ drive as “methyleneblue”. You will need to store the data on a floppy
disk so that you can take the disk to a different computer and analyze the data.
Now, configure the time channels by clicking Time Acquisition| Configure |Configure Time
Channels. Set the boxes as they appear in the picture below. Methylene blue absorbs at 655 nm.
You are now ready to begin acquiring data. Move to the absorbance screen by clicking the
absorbance button on the tool bar.
A
Remove the cuvette from the cuvette holder. Transfer the required amount of methylene blue into
the cuvette and invert the cuvette to mix the solutions. Wipe the outside of the cuvette with a
Kimwipe and return the cuvette to the cuvette holder.
On the computer, click Time Acquisition| Activate Time Acquisition. Then click Time Acquisition|
Start. In the status box underneath the spectrum, you can monitor how many acquisitions have
been made and how long you have been recording changes in absorbance.
The experiment will run for 200 seconds and then stop automatically. If no one is waiting for the
spectrophotometer, rinse you cuvette out with 0.10 M HCl and do the second experiment. Store
the results of the second experiment under the filename “methyleneblue2”.
DATA ANALYSIS:
To analyze your data, open your experimental file using Excel. The Text Import Wizard will appear.
Accept the default settings in the wizard by clicking Next each time. When the Finish button
appears, click it. A spreadsheet of data like this will appear.
Time (sec) Channel A
0.361
0.175
10.607
0.188
20.884
0.13
31.129
0.104
41.388
0.149
51.669
0.249
Analyze your data by graphing the raw data, the natural log of the data, and the inverse data.
Perform the linear regression of each graph to find the order of the reaction. Determine the
pseudo rate constant. Save this analysis as a new Excel file on your floppy disk. Print off the data
and the graph that shows the order of the reaction. A handout on how to make graphs like this is
available on the laboratory website.
Run a second trial using a different amount of methylene blue. Determine the order of the reaction
and the pseudo rate constant for the second experiment. Print off the data and the graph that
support your answers.
Compare the two rate constants and determine the percent difference between the two values.
Calculate the average pseudo rate constant. Write the rate law for this reaction using the pseudo
rate constant, k’.
LIBRARY PROJECT ON KINETICS
Name: ____________________________
Use the article on kinetics that you previously found to complete this assignment. Each student will
work independently on his or her individual articles to complete this assignment. It is possible that
your article cannot answer every question. If your article does not allow you to answer at least two
of the questions, you will need to find a new article for this project.
Read your article and answer the following questions. Use a highlighter pen to highlight the places
in the articles that answer these questions. Turn in both this sheet and your article next week before
the laboratory practical exam over kinetics.
Title of Article:
Authors:
Journal:
Chemical Reaction being studied:
QUESTIONS
1. Rate law for the reaction:
2. Order of reaction for each reactant:
3. Rate constant for the reaction:
4. Instrument/Equipment used to determine kinetics:
5. Method of data analysis (initial rates or integrated rate method):