P 1, 5, 9, 13, 15
advertisement
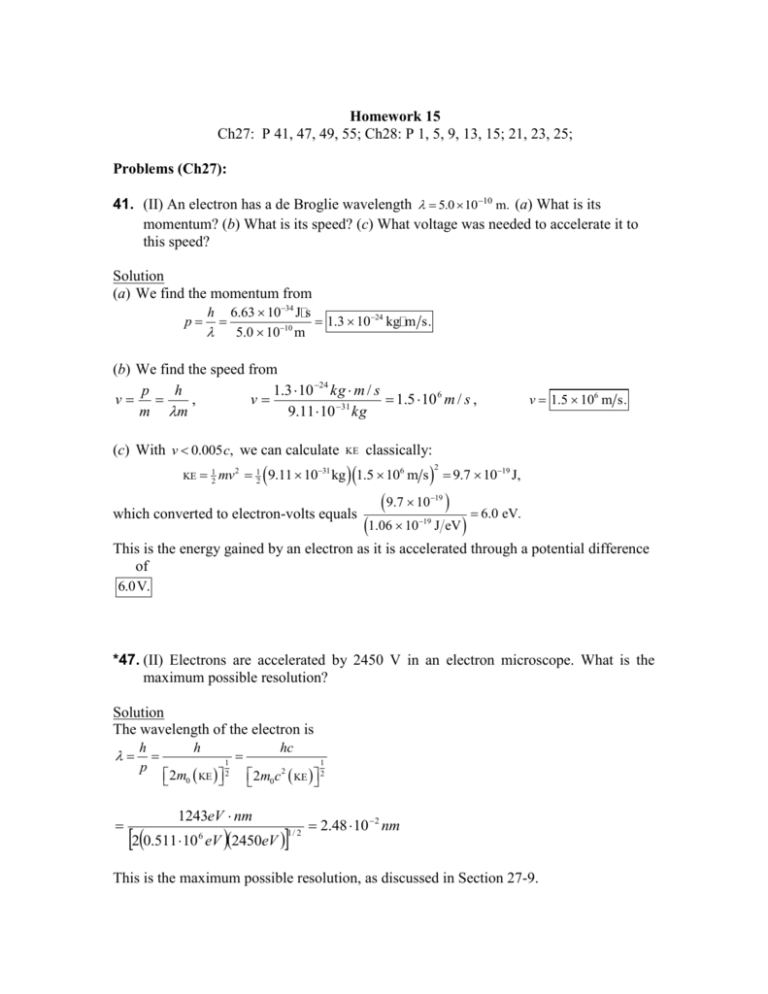
Homework 15 Ch27: P 41, 47, 49, 55; Ch28: P 1, 5, 9, 13, 15; 21, 23, 25; Problems (Ch27): 41. (II) An electron has a de Broglie wavelength 5.0 1010 m. (a) What is its momentum? (b) What is its speed? (c) What voltage was needed to accelerate it to this speed? Solution (a) We find the momentum from p h 6.63 1034 J s 1.3 1024 kg m s. 10 5.0 10 m (b) We find the speed from p h 1.3 10 24 kg m / s v , v 1.5 10 6 m / s , 31 m m 9.11 10 kg (c) With v 0.005 c, we can calculate KE KE classically: 12 mv2 12 9.11 1031 kg 1.5 106 m s 2 v 1.5 106 m s. 9.7 1019 J, 9.7 10 19 which converted to electron-volts equals 1.06 10 19 J eV 6.0 eV. This is the energy gained by an electron as it is accelerated through a potential difference of 6.0V. *47. (II) Electrons are accelerated by 2450 V in an electron microscope. What is the maximum possible resolution? Solution The wavelength of the electron is h p h 2m0 KE 1 2 hc 1 2m0 c 2 KE 2 1243eV nm 20.511 10 eV 2450eV 6 1/ 2 2.48 10 2 nm This is the maximum possible resolution, as discussed in Section 27-9. 49. (I) How much energy is needed to ionize a hydrogen atom in the n 2 state? Solution To ionize the atom means removing the electron, or raising it to zero energy: Eion 0 En 13.6 eV 13.6 eV n2 22 3.4 eV. 55. (II) What wavelength photon would be required to ionize a hydrogen atom in the ground state and give the ejected electron a kinetic energy of 10.0 eV? Solution The energy of the photon is hf Eion KE 13.6 eV 10.0 eV 23.6eV. We find the wavelength from hc 1243eV nm 52.7nm hf 23.6eV Problems (Ch28): 1. (II) The neutrons in a parallel beam, each having kinetic energy 401 eV, are directed through two slits 0.50 mm apart. How far apart will the interference peaks be on a screen 1.0 m away? [Hint: First find the wavelength of the neutron.] Solution We find the wavelength of the neutron from h p h 1 2m0 KE 2 6.63 10 J s kg 0.025eV 1.6 10 34 2 1.67 1027 19 J eV 1 2 1.81 1010 m. The peaks of the interference pattern are given by d sin n , n 1, 2, ... . and the positions on the screen are y L tan . For small angles, sin tan , so we have y Thus the separation is L 1.0m 1.81 10 10 m y 3.6 10 7 m 3 d 0.50 10 m nL . d 5. (I) An electron remains in an excited state of an atom for typically 10 8 s. What is the minimum uncertainty in the energy of the state (in eV)? Solution We find the minimum uncertainty in the energy of the state from 34 ÿ 1.055 10 J s E 1.1 1026 J 6.6 108 eV. 8 t 10 s 9. (II) An electron and a 140-g baseball are each traveling 150 m s measured to an accuracy of 0.055%. Calculate and compare the uncertainty in position of each. Solution The uncertainty in the velocity is 0.055 v 150m s 0.0825m s. 100 For the electron, we have 1.055 1034 J s ÿ x 1.4 103 m. m v 9.11 1031 kg 0.0825m s For the baseball, we have 1.055 1034 J s 9.1 1033 m. ÿ x m v 0.140kg 0.0825m s The uncertainty for the electron is greater by a factor of 1.5 1029. 13. (I) For n 6, what values can l have? Solution The value of 15. can range from 0 to n 1. Thus for n = 6, we have 0, 1, 2, 3, 4, 5. (I) How many electrons can be in the n 6, l 3 subshell? Solution The number of electrons in the subshell is determined by the value of . For each the value of m can range from to , or 2 1 values. For each of these there are two values of ms . Thus the total number for 3 is N 2 2 1 2 2 3 1 14 electrons. 21. (II) If a hydrogen atom has ml 3, what are the possible values of n, l, and m s ? Solution The value of m can range from to so we have 3. The value of can range from 0 to n 1. Thus we have n 1(minimum4). There are two values of ms : ms 12 , 12 . 23. (II) What is the full electron configuration in the ground state for elements with Z equal to (a) 27, (b) 36, (c) 38? [Hint: See the periodic table inside the back cover.] Solution (a) We start with hydrogen and fill the levels as indicated in the periodic table: 1s 2 2s 2 2 p 6 3s 2 3 p 6 3d 7 4s 2 . Note that the 4s 2 level is filled before the 3d level is started. (b) For Z 36 we have 1s 2 2s 2 2 p 6 3s 2 3 p 6 3d 10 4s 2 4 p 6 . (c) For Z 38 we have 1s 2 2s 2 2 p 6 3s 2 3 p 6 3d 10 4s 2 4 p 6 5s 2 . Note that the 5s 2 level is filled before the 4d level is started. 25. (II) A hydrogen atom is in the 6s state. Determine (a) the principal quantum number, (b) the energy of the state, (c) the orbital angular momentum and its quantum number l, and (d) the possible values for the magnetic quantum number. Solution (a) The principal quantum number is n 6. (b) The energy of the state is E6 13.6eV 13.6eV n2 (c) The “s” subshell has only: L ÿ (d) For each 62 0.378eV. 0. The magnitude of the angular momentum depends on 1 2 1.055 1034 J s 0 0 1 2 0. 1 1 the value of m can range from to : m 0.