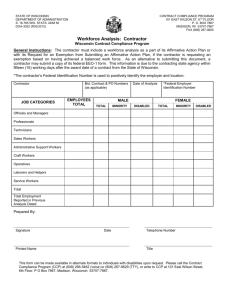
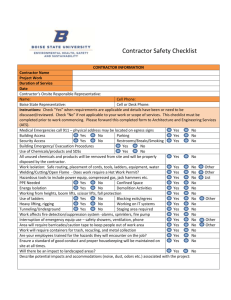
Complete Contract - Department of Management Services
advertisement

AMENDMENT NO. 4 State Term Contract Number 475-000-11-1 Medical and Dental Supplies This Amendment No. 4 (“Amendment”) effective on November 14, 2014, to the Medical and Dental Supplies State Term Contract No. 475-000-11-1 (“Contract”), between the State of Florida, Department of Management Services (“Department”) and Patterson Dental Supply, Inc. (“Contractor”) are collectively referred to herein as the “Parties.” All capitalized terms used herein shall have the meaning assigned to them in the Contract, unless otherwise defined herein. WHEREAS, the Contract was originally awarded to the Contractor on September 20, 2010, to expire on September 20, 2013, for the provision of Medical and Dental Supplies, pursuant to RFP No. 10-475-000-J; and WHEREAS, the Contract was renewed (Amendment 3) through November 14, 2014, pursuant to Section 287.057(13), Florida Statutes and Section 4.26 of the contract; and WHEREAS, in accordance with Section 287.057(13), Florida Statutes and section 4.26 of the Contract, upon mutual agreement, the parties may renew the Contract in whole or in part, for a period that may not exceed three years; and THEREFORE, in consideration of the mutual promises contained below, and other good and valuable consideration, receipt and sufficiency of which are hereby acknowledged, the Parties amend the Contract as follows: 1.0 Contract Renewal. Pursuant to Section 287.057(13), Florida Statutes, the Parties hereby execute its renewal option to expire not later than the end of the day on September 20, 2016. 2.0 Effect. Unless otherwise modified by this Amendment, all terms and conditions contained in the Contract shall continue in full force and effect. 3.0 Conflict. To the extent any of the terms of this Amendment conflict with the terms of the Contract, the terms of this Amendment shall control. AMENDMENT NO. 4 State Term Contract Number 475-000-11-1 Medical and Dental Supplies 4.0 Warrant of Authority. Each person signing this Amendment warrants that he or she is duly authorized to do so and to bind the respective party. State of Florida, Department of Management Services: Contractor: Patterson Dental Supply Inc By: ____________________________ By: ______________________________ Name: Kelley J. Scott Name: ___________________________ Title: Director of State Purchasing and Title: _____________________________ Chief Procurement Officer_____ Date: ___________________________ Date: ____________________________ AMENDMENT NO. 3 State Term Contract Number 475-000-11-1 Medical and Dental Supplies This Amendment No. 3 (“Amendment”) effective on September 20, 2014, to the Medical and Dental Supplies State Term Contract No. 475-000-11-1 (“Contract”), between the State of Florida, Department of Management Services (“Department”) and Binson’s Hospital Supplies (“Contractor”) are collectively referred to herein as the “Parties.” All capitalized terms used herein shall have the meaning assigned to them in the Contract, unless otherwise defined herein. WHEREAS, the Contract was originally awarded to the Contractor on September 20, 2010, to expire on September 20, 2013, for the provision of Medical and Dental Supplies, pursuant to RFP No. 10-475-000-J; and WHEREAS, the Contract was renewed (Amendment 2) through September 20, 2014, pursuant to Section 287.057(13), Florida Statutes and Section 4.26 of the contract; and WHEREAS, in accordance with Section 287.057(13), Florida Statutes and section 4.26 of the Contract, upon mutual agreement, the parties may renew the Contract in whole or in part, for a period that may not exceed three years; and THEREFORE, in consideration of the mutual promises contained below, and other good and valuable consideration, receipt and sufficiency of which are hereby acknowledged, the Parties amend the Contract as follows: 1.0 Contract Renewal. Pursuant to Section 287.057(13), Florida Statutes, the Parties hereby execute its renewal option for two years, to expire not later than the end of the day on September 20, 2016. 2.0 Contract Amendment or Modification. Per Section 4.42, paragraph 5.14 is added to the Contract and Section 7 of Amendment #1 is deleted in its entirety and replaced with “Core and Non-Core Pricing.” “5.14 Public Records. If, under this Contract, the Contractor is providing services and is acting on behalf of the Department as provided under section 119.011(2), Florida Statutes, the Contractor, subject to the terms of section 287.058(1)(c), Florida Statutes, and any other applicable legal and equitable remedies, shall: (a) Keep and maintain public records that ordinarily and necessarily would be required by the Department in order to perform the service. (b) Provide the public with access to public records on the same terms and conditions that the Department would provide the records and at a cost that does not exceed the cost provided in Chapter 119, Florida Statutes, or as otherwise provided by law. (c) Ensure that public records that are exempt or confidential and exempt from public records disclosure requirements are not disclosed except as authorized by law. AMENDMENT NO. 3 State Term Contract Number 475-000-11-1 Medical and Dental Supplies (d) Meet all requirements for retaining public records and transfer, at no cost, to the Department all public records in possession of the Contractor upon termination of the Contract and destroy any duplicate public records that are exempt or confidential and exempt from public records disclosure requirements. All records stored electronically must be provided to the Department in a format that is compatible with the information technology systems of the Department. The Department may unilaterally cancel this Contract for refusal by the contractor to comply with this section by not allowing public access to all documents, papers, letters, or other material made or received by the contractor in conjunction with the contract, unless the records are exempt from s. 24(a) of Art. I of the State Constitution and s. 119.07(1), Florida Statutes.” “7.0 Core and Non-Core Pricing. (a) Categories of Market Basket, Core and Balance of Line are hereby replaced by Core and Non-Core as defined below. (b) Pursuant to Section 6.3 “Scope” of the Contract, Core items are defined as all items listed on the current itemized Pricing Sheet, which includes any market basket and balance of line items with a specific individual price per item or unit. Non-Core items are defined as all other individual items the Vendor sells that includes a fixed discount percentage off for the duration of the contract or any renewals. Percentage off must be from the lowest publicly published list price, standard price, MSRP, and/or catalogue price. (c) Any item that is neither Core nor Non-Core is not available for sale under the Contract and shall not be included in any contract catalogue, including punch-out catalogues. Should an item become available for a fixed discount percentage during the term of the contract or any renewal period, the Vendor may request such items to be added to the Contract. It shall be at the sole discretion of the Department to allow additions to the Contract and/or added to any contract catalogue.” 3.0 Effect. Unless otherwise modified by this Amendment, all terms and conditions contained in the Contract shall continue in full force and effect. 4.0 Conflict. To the extent any of the terms of this Amendment conflict with the terms of the Contract, the terms of this Amendment shall control. AMENDMENT NO. 3 State Term Contract Number 475-000-11-1 Medical and Dental Supplies 5.0 Warrant of Authority. Each person signing this Amendment warrants that he or she is duly authorized to do so and to bind the respective party. State of Florida, Department of Management Services: Contractor: Binson’s Hospital Supplies By: ____________________________ By: ______________________________ Name: Kelley J. Scott Name: ___________________________ Title: Director of State Purchasing and Title: _____________________________ Chief Procurement Officer_____ Date: ___________________________ Date: ____________________________ AMENDMENT #2 TO Medical & Dental Supplies NO.: 475-000-11-1 This Amendment No. 2 (“Amendment”), is effective September 20, 2013, or the last date signed by both parties, to STC #475-000-11-1; Medical & Dental Supplies (“Agreement”), between the State of Florida, Department of Management Services (“Department”) and Binson’s Hospital Supplies (“Contractor”). The Department and Contractor are collectively referred to herein as the “Parties.” All capitalized terms used herein shall have the meaning assigned to them in the Agreement, unless otherwise defined herein. WHEREAS the Agreement was originally entered on September 20, 2010 with the Contractor for the provision of medical and dental supplies, and is scheduled to expire on September 20, 2013; and WHEREAS upon mutual agreement, the Department and the Contractor agree to amend the Agreement, in accordance with the its terms; and THEREFORE, in consideration of the mutual promises contained below, and other good and valuable consideration, receipt and sufficiency of which are hereby acknowledged, the Parties agree to the following: 1.0 Contract Amendment. Pursuant to section 287.057(13), Florida Statutes, STC #475-000-11-1; Medical & Dental Supplies is renewed for a period of one year effective September 20, 2013 and will expire September 20, 2014. 2.0 Effect. Unless otherwise modified by this Amendment, all terms and conditions contained in the Agreement shall continue in full force and effect. 3.0 Conflict. To the extent any of the terms of this Amendment conflict with the terms of the Agreement, the terms of this Amendment shall control. 4.0 Warrant of Authority. Each person signing this Amendment warrants that he or she is duly authorized to do so and to bind the respective party. 5.0 Successors and Assigns. This Amendment shall be binding upon and inure to the benefit of the successors and permitted assigns of the parties hereto. 6.0 Entire Agreement. Except as expressly modified by this Amendment, the Agreement shall be and remain in full force and effect in accordance with its terms and shall constitute the legal, valid, binding and enforceable obligations to the parties. This Amendment and the Agreement (including any written amendments thereto), collectively, are the complete agreement of the parties and supersede any prior agreements or representations, whether oral or written, with respect thereto. State of Florida, Department of Management Services: Binson’s Hospital Supplies: By: By: Name: Kelley J. Scott Name: Title: Director of State Purchasing and Chief Procurement Officer Title: Date: Date: AMENDMENT #1 TO Medical & Dental Supplies NO.: 475-000-11-1 This Amendment #1 (Amendment), effective as of September 20, 2013 or on the last date on which it is signed by all parties, whichever is later, to the state term contract #475-000-11-1; Medical & Dental Supplies (Contract) effective as of September 21, 2010, between the State of Florida, Department of Management Services (Department) and Binson’s Hospital Supplies (Contractor). Department and Contractor are collectively referred to herein as the Parties. All capitalized terms used herein shall have the meaning assigned to them in the Agreement, unless otherwise defined herein. WHEREAS, Pursuant to Section 4.42 the Parties wish to amend certain terms and conditions of the Contract; NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties hereby agree as follows: 1.0 The content of 5.6 is hereby amended to read as follows: “5.6 Punch-out Catalog 5.6.1 The Contractor hereby agrees to cooperate with the Department and MyFloridaMarketPlace (MFMP) and any authorized agent or successor entity to MFMP in the event the Department selects this statewide contract to be exhibited on MFMP. At a minimum, the Contractor agrees to the following: a. Upon the Department’s’ request, to deliver a punch-out catalog within ninety (90) days of written request. The punch-out catalog content must be limited to the Contractor’s statewide contract offering. b. In addition to the Contractor’s punch-out catalog in MFMP, the Contractor shall provide a specific online ordering portal and product catalog (referred to as the online catalog) to the State of Florida containing only State of Florida items and prices. The contractor will, within ninety (90) days of this amendment, make available the online catalog that contains only items that are in the scope of the awarded contract. For each item, the following information shall be provided at a minimum: item description, manufacturer name, manufacturer part number, unit of measure, list price and contract price, and item image. c. The Contractor agrees to meet the following requirements: i. Catalog must contain the most current pricing, including all applicable administrative fees and or discounts, as well as the most up-to-date product/service offering the Contractor is authorized to provide in accordance with the statewide contract; and ii. The accuracy of the catalog must be maintained by Contractor throughout the duration of the statewide contract; and iii. The catalog must include a State-specific contract identification number; and iv. The catalog must include detailed product line item descriptions; and v. The catalog must include pictures when possible; and vi. The catalog must include any additional Department content requirements. d. Contractor agrees that the Department controls which statewide contracts appear in MFMP and that the Department may elect at any time to remove any Contractor’s offering from MFMP. e. Contractor must be able to accept Purchase Orders via fax, e-mail, cXML or EDI INT AS 12. 5.6.2 e-Invoicing The Contractor shall supply electronic invoices in lieu of paper-based invoices for those transactions processed through the MFMP within ninety (90) days from the effective date of the amendment. Electronic invoices shall be submitted to the agency through the Ariba Supplier Network (ASN) in one of three mechanisms as listed below: a. cXML (commerce eXtensible Markup Language) This standard establishes the data contents required for invoicing via cXMLwithin the context of an electronic environment. This transaction set can be used for invoicing via the ASN for catalog and non-catalog goods and services. The cXML format is the Ariba preferred method for eInvoicing. b. EDI (Electronic Data Interchange) This standard establishes the data contents of the Invoice Transaction Set (810) for use within the context of an Electronic Data Interchange (EDI) environment. This transaction set can be used for invoicing via the ASN for catalog and non-catalog goods and services. c. PO Flip via ASN The online process allows Contractors to submit invoices via the ASN for catalog and non-catalog goods and services. Contractors have the ability to create an invoice directly from their Inbox in their ASN account by simply “flipping” the PO into an invoice. This option does not require any special software or technical capabilities. For the purposes of this section, the Contractor warrants and represents that it is authorized and empowered to and hereby grants the State of Florida and the third party provider of MFMP, a state contractor, the right and license to use, reproduce, transmit, distribute, and publicly display within the system the information outlined above. In addition, the Contractor warrants and represents that it is authorized and empowered to and hereby grants the State and the third party provider the right and license to reproduce and display within the system the Contractor’s trademarks, system marks, logos, trade dress, or other branding designation that identifies the products made available by the Contractor under the contract.” The Contractor will work with the MFMP management team to obtain specific requirements for the electronic invoicing. 2.0 Quarterly Sales Reporting Requirements The Contractor agrees to submit a sales report on a Quarterly basis. Reporting periods coincide with the State Fiscal Year: Quarter 1- (July‐September) Quarter 2 - (October‐December) Quarter 3 ‐ (January‐March) Quarter 4 - (April‐June) Report due October 30 Report due January 31 Report due May 1 Report due July 30 Each Quarterly Sales Report must be in Excel format in accordance with the Department’s “Contract Quarterly Report”. (Attachment A) Failure to provide quarterly sales reports, including no sales, within thirty (30) calendar days following the end of each quarter (January, April, July and October) and/or contract year may result in the contract supplier being found in default and cancellation of the contract by the Department. 3.0 Business Review Meetings The Department reserves the right to schedule business review meetings as frequently as necessary. The Department will provide the format for the Contractor’s agenda. Prior to the meeting, the Contractor shall submit the completed agenda to the Department for review and acceptance. The Contractor shall address the agenda items and any of the Department’s additional concerns at the meeting. Failure to comply with this section may result in the Contractor being found in default and contract termination. 4.0 Employment Verification Contractor agrees that it will enroll and participate in the federal E-Verify Program for Employment Verification under the terms provided in the “Memorandum of Understanding” governing the program. Contractor further agrees to provide to the Agency, within thirty (30) days of the effective date of this Amendment, documentation of such enrollment in the form of a copy of the E-Verify “Edit Company Profile” screen,” which contains proof of enrollment in the E-Verify Program (this page can be accessed from the “Edit Company Profile” link on the left navigation menu of the E-Verify employer’s homepage). Contractor further agrees that it will require each subcontractor that performs work under this Contract to enroll and participate in the E-Verify Program within ninety (90) days of the effective date of this Amendment or within ninety (90) days of the effective date of the contract between the Contractor and the subcontractor, whichever is later. The Contractor shall obtain from the subcontractor(s) a copy of the “Edit Company Profile” screen indicating enrollment in the E-Verify Program and make such record(s) available to the Agency upon request. Contractor further agrees to maintain records of its participation and compliance with the provisions of the E-Verify program, including participation by its subcontractors as provided above, and to make such records available to the Agency or other authorized state entity consistent with the terms of the Memorandum of Understanding. 5.0 Scrutinized Company List In executing this Amendment, Contractor certifies that it is not listed on either the Scrutinized Companies with Activities in Sudan List or the Scrutinized Companies with Activities in the Iran Petroleum Energy Sector List, created pursuant to section 215.473, Florida Statutes. Pursuant to section 287.135(5), F.S., Contractor agrees the Department may immediately terminate this contract for cause if the Contractor is found to have submitted a false certification or if Contractor is placed on the Scrutinized Companies with Activities in Sudan List or the Scrutinized Companies with Activities in the Iran Petroleum Energy Sector List during the term of the contract. (Attachment B) 6.0 Preferred Pricing The Contractor agrees to submit to Customer at least annually an affidavit from an authorized representative attesting that the Contractor is in compliance with the Best Pricing provision in Section 4(b) of form PUR 1000. (Attachment C) 7.0 Core and Marketbasket Pricing The Contractor agrees to provide the items contained within the attached Pricing Sheet to reflect newly-aligned core and marketbasket lists as well as discounts on Contractor’s remaining balance of line. (Attachment D) 8.0 Automatic Electronic Defibrillators and related accessories The parties have agreed to make Automatic Electronic Defibrillators and related accessories previously available under the Oklahoma/NASPO agreement SW300 now available under this contract. The Contractor agrees to provide the Automatic Electronic Defibrillators and related accessories at or below the prices depicted in Attachment D. 9.0 Commitment to Diversity in Government Contracting The State of Florida is committed to supporting its diverse business industry and population through ensuring participation by minority-, women-, wartime-, and servicedisabled veteran business enterprises in the economic life of the State. The State of Florida Mentor Protégé Program connects minority, women, wartime, and servicedisabled veteran business enterprises with private corporations for business development mentoring. We strongly encourage firms doing business with the State of Florida to consider this initiative. For more information on the Mentor Protégé Program, please contact the Office of Supplier Diversity at (850) 487-0915 or osdhelp@dms.myflorida.com. Upon request, the Contractor shall report to the Department spend with certified and other minority business enterprises. These reports will include the period covered, the name, minority code and Federal Employer Identification Number of each minority vendor utilized during the period, commodities and services provided by the minority business enterprise, and the amount paid to each minority vendor on behalf of each purchasing agency ordering under the terms of this Contract. 10.0 Conflict To the extent any of the terms of this Amendment conflict with the terms of the Contract, the terms of this Amendment shall control. 11.0 Warrant of Authority Each person signing this Amendment warrants that he or she is duly authorized to do so and to bind the respective party. 12.0 Effect Unless otherwise modified by this Amendment, all terms and conditions contained in the Contract shall continue in full force and effect. State of Florida, Department of Services: Binson’s Hospital Supplies: : Management By:____________________________ By:_____________________________ Name: Kelley J. Scott Name:___________________________ Title: Director of Purchasing and Chief Procurement Officer Title:____________________________ Date:_________________________ Date:____________________________ CERTIFICATION OF CONTRACT Title: Medical and Dental Supplies Contract Number: 475-000-11-1 RFP Number: 10-475-000-J Effective: September 20, 2010 through September 19, 2013 Supersedes: 475-000-05-1 Contractors: Medical Supplies; Binson Hospital Supplies (A) Henry Schein (A) Physicians Sales and Services (A) Dental Supplies; Henry Schein (A) Patterson Dental (A) A) AUTHORITY - Upon affirmative action taken by the State of Florida Department of Management Services, a contract has been executed between the State of Florida and the designated contractors. B) EFFECT - This contract was entered into to provide economies in the purchase of Medical and Dental Supplies by all State of Florida agencies and institutions. Therefore, in compliance with Section 287.042, Florida Statutes, all purchases of these commodities shall be made under the terms, prices, and conditions of this contract and with the suppliers specified. C) ORDERING INSTRUCTIONS - All purchase orders shall be issued in accordance with the attached ordering instructions. Purchaser shall order at the prices indicated, exclusive of all Federal, State and local taxes. All contract purchase orders shall show the State Purchasing contract number, product number, quantity, description of item, with unit prices extended and purchase order totaled. (This requirement may be waived when purchase is made by a blanket purchase order.) D) CONTRACTOR PERFORMANCE - Agencies shall report any vendor failure to perform according to the requirements of this contract on Complaint to Vendor, form PUR 7017. Should the vendor fail to correct the problem within a prescribed period of time, then form PUR 7029, Request for Assistance, is to be filed with this office. E) SPECIAL AND GENERAL CONDITIONS - Special and general conditions are enclosed for your information. Any restrictions accepted from the supplier are noted on the ordering instructions. ___ _____________________________ Authorized Signature DSP/ds Attachments (date) REQUEST FOR PROPOSAL (RFP) FOR MEDICAL AND DENTAL SUPPLIES RFP NO. 10-475-000-J RFP ISSUE DATE: JULY 13, 2010 RESPONSES DUE: AUGUST 18, 2010 REFER ALL INQUIRIES TO: DONNA SMITH, PURCHASING ANALYST STATE OF FLORIDA DEPARTMENT OF MANAGEMENT SERVICES, DIVISION OF STATE PURCHASING 4050 ESPLANADE WAY, SUITE 360 TALLAHASSEE, FL 32399 PHONE: 850-488-8855 FAX: 850-414-6122 EMAIL: DONNA.SMITH@DMS.MYFLORIDA.COM RFP No. 10-475-000-J Medical and Dental Supplies Page 1 Table of Contents SECTION 1.0 INTRODUCTION 1.1 Introduction and Overview 1.2 Event Timeline SECTION 2.0 General Instructions to Respondents (PUR 1001) 2.1. Definitions. 2.2. General Instructions. 2.3. Electronic Submission of Responses. 2.4. Terms and Conditions. 2.5. Questions. 2.6. Conflict of Interest. 2.7. Convicted Vendors. 2.8. Discriminatory Vendors. 2.9. Respondent’s Representation and Authorization. 2.10. Manufacturer’s Name and Approved Equivalents. 2.11. Performance Qualifications. 2.12. Public Opening. 2.13. Electronic Posting of Notice of Intended Award. 2.14. Firm Response. 2.15. Clarifications/Revisions. 2.16. Minor Irregularities/Right to Reject. 2.17. Contract Formation. 2.18. Contract Overlap. 2.19. Public Records. 2.20. Protests. 2.21. Limitation on Vendor Contact with Agency during Solicitation Period Section 3.0 Special Instructions To Respondents 3.1 Contact Person 3.2 Definitions 3.3 Firm Response 3.4 Electronic Posting of Award 3.5 Order of Precedence 3.6 Submittal of Response 3.7 Addendums to the Solicitation Documents 3.8 Submittal Content 3.9 Estimated Sales 3.10 Evaluation and Selection Process 3.11 Basis for Award 3.12 Price 3.13 Lobbying 3.14 Documentation Becomes the Property of the State 3.15 Notice to Contractor 3.16 Method of Payment 3.17 Environmental Considerations 3.18 Certification of Drug-Free Workplace Program 3.19 Public Entity Crimes RFP No. 10-475-000-J Medical and Dental Supplies Page 2 SECTION 4.0 GENERAL CONTRACT CONDITIONS (STATE OF FLORIDA FORM PUR 1000) 4.1 Definitions. 4.2 Purchase Orders. 4.3 Product Version. 4.4 Price Changes Applicable only to Term Contracts. 4.5 Additional Quantities. 4.6 Packaging. 4.7 Inspection at Contractor’s Site. 4.8 Safety Standards. 4.9 Americans with Disabilities Act. 4.10 Literature. 4.11 Transportation and Delivery. 4.12 Installation. 4.13 Risk of Loss. 4.14 Transaction Fee. 4.15 Invoicing and Payment. 4.16 Taxes. 4.17 Governmental Restrictions. 4.18 Lobbying and Integrity. 4.19 Indemnification. 4.20 Limitation of Liability. 4.21 Suspension of Work. 4.22 Termination for Convenience. 4.23 Termination for Cause. 4.24 Force Majeure, Notice of Delay, and No Damages for Delay. 4.25 Changes. 4.26 Renewal. 4.27 Purchase Order Duration. 4.28 Advertising. 4.29 Assignment. 4.30 Antitrust Assignment 4.31 Dispute Resolution. 4.32 Employees, Subcontractors, and Agents. 4.33 Security and Confidentiality. 4.34 Contractor Employees, Subcontractors, and Other Agents. 4.35 Insurance Requirements. 4.36 Warranty of Authority. 4.37 Warranty of Ability to Perform. 4.38 Notices. 4.39 Leases and Installment Purchases. 4.40 Prison Rehabilitative Industries and Diversified Enterprises, Inc. (PRIDE). 4.41 Products Available from the Blind or Other Handicapped. 4.42 Modification of Terms. 4.43 Cooperative Purchasing. 4.44 Waiver. 4.45 Annual Appropriations. 4.46 Execution in Counterparts. 4.47 Severability. RFP No. 10-475-000-J Medical and Dental Supplies Page 3 SECTION 5.0 SPECIAL CONTRACT CONDITIONS 5.1 Customer Service Support 5.2 Insurance Requirements 5.3 Contract Revisions 5.4 Compliance with Laws 5.5 Price Escalation / De-Escalation 5.6 Punch out Catalogues and e-Invoicing 5.7 Emergency Contact 5.8 Performance and Payment Bonds 5.9 Contractor Performance 5.10 Delays and Complaints 5.11 Sales Requirements 5.12 Contract Sales Summary Report 5.13 State Objectives SECTION 6.0 TECHNICAL SPECIFICATIONS 6.1 Purpose 6.2 Experience and Certifications 6.3 Scope SECTION 7.0 FORMS AND ATTACHMENTS Attachment 1: Proposal Checklist Form Attachment 2: Reference Summaries Attachment 3: Experience Certification Form Attachment 4: Respondents Capability Form with District Map Attachment 5: Ordering Instructions Attachment 6: Certification of Drug Free Workplace Program Attachment 7: Excel® Price Sheet RFP No. 10-475-000-J Medical and Dental Supplies Page 4 SECTION 1 INTRODUCTION 1.1 Introduction and Overview The Department of Management Services, Division of State Purchasing, invites interested companies to submit bids in accordance with these solicitation documents. The purpose of this Request for Proposal (RFP) is to establish a three (3) year state term contract for Medical and Dental Supplies with the potential for renewal in accordance with F.S. 287.057(1)(a) and if deemed in the best interest of the State. The contract resulting from this solicitation, if any, will provide efficiency and economy for state agencies and eligible users, by providing a preferred price schedule from one or more manufacturers and/or authorized distributors of medical and dental supplies. It is intended that the resultant contract, if any, be a multiple award; however, the State does reserve the right to award the entire contract, if any, to a single Contractor. This RFP and subsequent contract, if any, does not guarantee any level of business to the awarded supplier(s). The awarded supplier(s) will receive Direct Orders (DO), or Purchase Orders (PO) or credit card orders directly from the Customer. This solicitation includes the following Commodity Code (description is to the right) and components thereof; 475-XXX 260-XXX 1.2 Hospital Supplies Dental Equipment and Supplies Event Timeline Respondents should review and become familiar with the Event Timeline. The dates and times of each activity within the Timeline may be subject to change. It is the responsibility of the Respondent to check for any changes. All changes to the Timeline will be made through an addendum to this solicitation and posted within the Vendor Bid System (http://vbs.dms.state.fl.us/vbs/main_menu) and MyFloridaMarketPlace http://dms.myflorida.com/business_operations/state_purchasing/myflorida_marketplace/vendors /vendor_tools/sourcing_solicitations sourcing tool. A non-mandatory bid conference will address general questions related to the submittal process. Any questions related to specifications or requirements of the solicitation shall be addressed through the question and answer process. (See Section 2.5) Potential Respondents may call 1-888-808-6959 at 3:30 on July 16, 2010 to participate. Use Conference Code 4888855. Event 1 2 Post notice of solicitation on Vendor Bid System (VBS) and MyFloridaMarketPlace sourcing tool. Solicitation will initially be in “preview” status where offerors can view/download all information and ask questions, but CANNOT input or submit responses. Bid Conference – Non Mandatory – 1:00 to 2:00 PM Call in with your questions RFP No. 10-475-000-J Medical and Dental Supplies Event Date July 13, 2010 July 15, 2010 Page 5 6 regarding the use of MFMP. 4050 Esplanade Way, Tallahassee, FL 32399. Potential Respondents do not need to travel to Tallahassee. Teleconferencing is provided. Please call 888-808-6959 and use Conference Code 4888855 Deadline to submit final questions via the MyFloridaMarketPlace sourcing tool Q&A Board Post answers to questions as an Addendum to the solicitation on Vendor Bid System (VBS) and MyFloridaMarketPlace sourcing tool. Deadline to submit RFP response, including all required documents, in CD format. Responses are due no Later than 2:00 PM EDT. Evaluators Meeting and Award recommendation 6 7 Notice of Intent to Award posted on Vendor Bid System (VBS) Contract formation date 3 4 5 July 20, 2010 July 23, 2010 August 18, 2010 To be Announced via Amendment TBD Per Section 2.17 The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 6 State of Florida Section 2 PUR 1001 General Instructions to Respondents Contents 1. Definitions. 2. General Instructions. 3. Electronic Submission of Responses. 4. Terms and Conditions. 5. Questions. 6. Conflict of Interest. 7. Convicted Vendors. 8. Discriminatory Vendors. 9. Respondent’s Representation and Authorization. 10. Manufacturer’s Name and Approved Equivalents. 11. Performance Qualifications. 12. Public Opening. 13. Electronic Posting of Notice of Intended Award. 14. Firm Response. 15. Clarifications/Revisions. 16. Minor Irregularities/Right to Reject. 17. Contract Formation. 18. Contract Overlap. 19. Public Records. 20. Protests. 21. Limitation on Vendor Contact with Agency During Solicitation Period 1. Definitions. The definitions found in s. 60A-1.001, F.A.C. shall apply to this agreement. The following additional terms are also defined: (a) "Buyer" means the entity that has released the solicitation. The “Buyer” may also be the “Customer” as defined in the PUR 1000 if that entity meets the definition of both terms. (b) "Procurement Officer" means the Buyer's contracting personnel, as identified in the Introductory Materials. (c) "Respondent" means the entity that submits materials to the Buyer in accordance with these Instructions. (d) "Response" means the material submitted by the respondent in answering the solicitation. (e) "Timeline" means the list of critical dates and actions included in the Introductory Materials. 2. General Instructions. Potential respondents to the solicitation are encouraged to carefully review all the materials contained herein and prepare responses accordingly. 3. Electronic Submission of Responses. Respondents are required to submit responses electronically. For this purpose, all references herein to signatures, signing requirements, or other required acknowledgments hereby include electronic signature by means of clicking the "Submit Response" button (or other similar symbol or process) attached to or logically associated with the response created by the respondent within MyFloridaMarketPlace. The respondent agrees that the action of electronically submitting its response constitutes: an electronic signature on the response, generally, RFP No. 10-475-000-J Medical and Dental Supplies Page 7 an electronic signature on any form or section specifically calling for a signature, and an affirmative agreement to any statement contained in the solicitation that requires a definite confirmation or acknowledgement. 4. Terms and Conditions. All responses are subject to the terms of the following sections of this solicitation, which, in case of conflict, shall have the order of precedence listed: Technical Specifications, Special Conditions and Instructions, Instructions to Respondents (PUR 1001), General Conditions (PUR 1000), and Introductory Materials. The Buyer objects to and shall not consider any additional terms or conditions submitted by a respondent, including any appearing in documents attached as part of a respondent’s response. In submitting its response, a respondent agrees that any additional terms or conditions, whether submitted intentionally or inadvertently, shall have no force or effect. Failure to comply with terms and conditions, including those specifying information that must be submitted with a response, shall be grounds for rejecting a response. 5. Questions. Respondents shall address all questions regarding this solicitation to the Procurement Officer. Questions must be submitted via the Q&A Board within MyFloridaMarketPlace and must be RECEIVED NO LATER THAN the time and date reflected on the Timeline. Questions shall be answered in accordance with the Timeline. All questions submitted shall be published and answered in a manner that all respondents will be able to view. Respondents shall not contact any other employee of the Buyer or the State for information with respect to this solicitation. Each respondent is responsible for monitoring the MyFloridaMarketPlace site for new or changing information. The Buyer shall not be bound by any verbal information or by any written information that is not contained within the solicitation documents or formally noticed and issued by the Buyer's contracting personnel. Questions to the Procurement Officer or to any Buyer personnel shall not constitute formal protest of the specifications or of the solicitation, a process addressed in paragraph 19 of these Instructions. 6. Conflict of Interest. This solicitation is subject to chapter 112 of the Florida Statutes. Respondents shall disclose with their response the name of any officer, director, employee or other agent who is also an employee of the State. Respondents shall also disclose the name of any State employee who owns, directly or indirectly, an interest of five percent (5%) or more in the respondent or its affiliates. 7. Convicted Vendors. A person or affiliate placed on the convicted vendor list following a conviction for a public entity crime is prohibited from doing any of the following for a period of 36 months from the date of being placed on the convicted vendor list: submitting a bid on a contract to provide any goods or services to a public entity; submitting a bid on a contract with a public entity for the construction or repair of a public building or public work; submitting bids on leases of real property to a public entity; being awarded or performing work as a contractor, supplier, subcontractor, or consultant under a contract with any public entity; and transacting business with any public entity in excess of the Category Two threshold amount ($35,000) provided in section 287.017 of the Florida Statutes. RFP No. 10-475-000-J Medical and Dental Supplies Page 8 8. Discriminatory Vendors. An entity or affiliate placed on the discriminatory vendor list pursuant to section 287.134 of the Florida Statutes may not: submit a bid on a contract to provide any goods or services to a public entity; submit a bid on a contract with a public entity for the construction or repair of a public building or public work; submit bids on leases of real property to a public entity; be awarded or perform work as a contractor, supplier, sub-contractor, or consultant under a contract with any public entity; or transact business with any public entity. 9. Respondent’s Representation and Authorization. In submitting a response, each respondent understands, represents, and acknowledges the following (if the respondent cannot so certify to any of following, the respondent shall submit with its response a written explanation of why it cannot do so). The respondent is not currently under suspension or debarment by the State or any other governmental authority. To the best of the knowledge of the person signing the response, the respondent, its affiliates, subsidiaries, directors, officers, and employees are not currently under investigation by any governmental authority and have not in the last ten (10) years been convicted or found liable for any act prohibited by law in any jurisdiction, involving conspiracy or collusion with respect to bidding on any public contract. Respondent currently has no delinquent obligations to the State, including a claim by the State for liquidated damages under any other contract. The submission is made in good faith and not pursuant to any agreement or discussion with, or inducement from, any firm or person to submit a complementary or other noncompetitive response. The prices and amounts have been arrived at independently and without consultation, communication, or agreement with any other respondent or potential respondent; neither the prices nor amounts, actual or approximate, have been disclosed to any respondent or potential respondent, and they will not be disclosed before the solicitation opening. The respondent has fully informed the Buyer in writing of all convictions of the firm, its affiliates (as defined in section 287.133(1)(a) of the Florida Statutes), and all directors, officers, and employees of the firm and its affiliates for violation of state or federal antitrust laws with respect to a public contract for violation of any state or federal law involving fraud, bribery, collusion, conspiracy or material misrepresentation with respect to a public contract. This includes disclosure of the names of current employees who were convicted of contract crimes while in the employ of another company. Neither the respondent nor any person associated with it in the capacity of owner, partner, director, officer, principal, investigator, project director, manager, auditor, or position involving the administration of federal funds: o Has within the preceding three years been convicted of or had a civil judgment rendered against them or is presently indicted for or otherwise criminally or civilly charged for: commission of fraud or a criminal offense in connection with obtaining, attempting to obtain, or performing a federal, state, or local government transaction or public contract; violation of federal or state antitrust statutes; or commission of embezzlement, theft, forgery, bribery, falsification or destruction of records, making false statements, or receiving stolen property; or o Has within a three-year period preceding this certification had one or more federal, state, or local government contracts terminated for cause or default. RFP No. 10-475-000-J Medical and Dental Supplies Page 9 The product offered by the respondent will conform to the specifications without exception. The respondent has read and understands the Contract terms and conditions, and the submission is made in conformance with those terms and conditions. If an award is made to the respondent, the respondent agrees that it intends to be legally bound to the Contract that is formed with the State. The respondent has made a diligent inquiry of its employees and agents responsible for preparing, approving, or submitting the response, and has been advised by each of them that he or she has not participated in any communication, consultation, discussion, agreement, collusion, act or other conduct inconsistent with any of the statements and representations made in the response. The respondent shall indemnify, defend, and hold harmless the Buyer and its employees against any cost, damage, or expense which may be incurred or be caused by any error in the respondent’s preparation of its bid. All information provided by, and representations made by, the respondent are material and important and will be relied upon by the Buyer in awarding the Contract. Any misstatement shall be treated as fraudulent concealment from the Buyer of the true facts relating to submission of the bid. A misrepresentation shall be punishable under law, including, but not limited to, Chapter 817 of the Florida Statutes. 10. Manufacturer’s Name and Approved Equivalents. Unless otherwise specified, any manufacturers’ names, trade names, brand names, information or catalog numbers listed in a specification are descriptive, not restrictive. With the Buyer’s prior approval, the Contractor may provide any product that meets or exceeds the applicable specifications. The Contractor shall demonstrate comparability, including appropriate catalog materials, literature, specifications, test data, etc. The Buyer shall determine in its sole discretion whether a product is acceptable as an equivalent. 11. Performance Qualifications. The Buyer reserves the right to investigate or inspect at any time whether the product, qualifications, or facilities offered by Respondent meet the Contract requirements. Respondent shall at all times during the Contract term remain responsive and responsible. In determining Respondent’s responsibility as a vendor, the agency shall consider all information or evidence which is gathered or comes to the attention of the agency which demonstrates the Respondent’s capability to fully satisfy the requirements of the solicitation and the contract. Respondent must be prepared, if requested by the Buyer, to present evidence of experience, ability, and financial standing, as well as a statement as to plant, machinery, and capacity of the respondent for the production, distribution, and servicing of the product bid. If the Buyer determines that the conditions of the solicitation documents are not complied with, or that the product proposed to be furnished does not meet the specified requirements, or that the qualifications, financial standing, or facilities are not satisfactory, or that performance is untimely, the Buyer may reject the response or terminate the Contract. Respondent may be disqualified from receiving awards if respondent, or anyone in respondent’s employment, has previously failed to perform satisfactorily in connection with public bidding or contracts. This paragraph shall not mean or imply that it is obligatory upon the Buyer to make an investigation either before or after award of the Contract, but should the Buyer elect to do so, respondent is not relieved from fulfilling all Contract requirements. RFP No. 10-475-000-J Medical and Dental Supplies Page 10 12. Public Opening. Responses shall be opened on the date and at the location indicated on the Timeline. Respondents may, but are not required to, attend. The Buyer may choose not to announce prices or release other materials pursuant to s. 119.071(1)(b), Florida Statutes. Any person requiring a special accommodation because of a disability should contact the Procurement Officer at least five (5) workdays prior to the solicitation opening. If you are hearing or speech impaired, please contact the Buyer by using the Florida Relay Service at (800) 9558771 (TDD). 13. Electronic Posting of Notice of Intended Award. Based on the evaluation, on the date indicated on the Timeline the Buyer shall electronically post a notice of intended award at http://fcn.state.fl.us/owa_vbs/owa/vbs_www.main_menu. If the notice of award is delayed, in lieu of posting the notice of intended award the Buyer shall post a notice of the delay and a revised date for posting the notice of intended award. Any person who is adversely affected by the decision shall file with the Buyer a notice of protest within 72 hours after the electronic posting. The Buyer shall not provide tabulations or notices of award by telephone. 14. Firm Response. The Buyer may make an award within sixty (60) days after the date of the opening, during which period responses shall remain firm and shall not be withdrawn. If award is not made within sixty (60) days, the response shall remain firm until either the Buyer awards the Contract or the Buyer receives from the respondent written notice that the response is withdrawn. Any response that expresses a shorter duration may, in the Buyer's sole discretion, be accepted or rejected. 15. Clarifications/Revisions. Before award, the Buyer reserves the right to seek clarifications or request any information deemed necessary for proper evaluation of submissions from all respondents deemed eligible for Contract award. Failure to provide requested information may result in rejection of the response. 16. Minor Irregularities/Right to Reject. The Buyer reserves the right to accept or reject any and all bids, or separable portions thereof, and to waive any minor irregularity, technicality, or omission if the Buyer determines that doing so will serve the State’s best interests. The Buyer may reject any response not submitted in the manner specified by the solicitation documents. 17. Contract Formation. The Buyer shall issue a notice of award, if any, to successful respondent(s), however, no contract shall be formed between respondent and the Buyer until the Buyer signs the Contract. The Buyer shall not be liable for any costs incurred by a respondent in preparing or producing its response or for any work performed before the Contract is effective. 18. Contract Overlap. Respondents shall identify any products covered by this solicitation that they are currently authorized to furnish under any state term contract. By entering into the Contract, a Contractor authorizes the Buyer to eliminate duplication between agreements in the manner the Buyer deems to be in its best interest. 19. Public Records. Article 1, section 24, Florida Constitution, guarantees every person access to all public records, and Section 119.011, Florida Statutes, provides a broad definition of public record. As such, all responses to a competitive solicitation are public records unless exempt by law. Any respondent claiming that its response contains information that is exempt from the public records law shall clearly segregate and mark that information and provide the specific statutory citation for such exemption. RFP No. 10-475-000-J Medical and Dental Supplies Page 11 20. Protests. Any protest concerning this solicitation shall be made in accordance with sections 120.57(3) and 287.042(2) of the Florida Statutes and chapter 28-110 of the Florida Administrative Code. Questions to the Procurement Officer shall not constitute formal notice of a protest. It is the Buyer's intent to ensure that specifications are written to obtain the best value for the State and that specifications are written to ensure competitiveness, fairness, necessity and reasonableness in the solicitation process. Section 120.57(3) (b), F.S. and Section 28-110.003, Fla. Admin. Code require that a notice of protest of the solicitation documents shall be made within seventy-two hours after the posting of the solicitation. Section 120.57(3) (a), F.S. requires the following statement to be included in the solicitation: "Failure to file a protest within the time prescribed in section 120.57(3), Florida Statutes, shall constitute a waiver of proceedings under Chapter 120, Florida Statutes." Section 28-110.005, Fla. Admin. Code requires the following statement to be included in the solicitation: "Failure to file a protest within the time prescribed in Section 120.57(3), Florida Statutes, or failure to post the bond or other security required by law within the time allowed for filing a bond shall constitute a waiver of proceedings under Chapter 120, Florida Statutes.” 21. Limitation on Vendor Contact with Agency During Solicitation Period. Respondents to this solicitation or persons acting on their behalf may not contact, between the release of the solicitation and the end of the 72-hour period following the agency posting the notice of intended award, excluding Saturdays, Sundays, and state holidays, any employee or officer of the executive or legislative branch concerning any aspect of this solicitation, except in writing to the procurement officer or as provided in the solicitation documents. Violation of this provision may be grounds for rejecting a response. RFP No. 10-475-000-J Medical and Dental Supplies Page 12 SECTION 3 Special Instructions to Respondents NOTE: SPECIAL INSTRUCTIONS TO RESPONDENTS CONTAINED IN SECTION 3 WILL SUPERSEDE AND SUPPLEMENT GENERAL INSTRUCTIONS TO RESPONDENTS (PUR 1001) CONTAINED IN SECTION 2. 3.1 Contact Person Refer contract inquiries to: Donna Smith, Purchasing Analyst Department of Management Services, Division of State Purchasing 4050 Esplanade Way, Suite 360 Tallahassee, FL 32399-0950 Phone: 850-488-8855 Fax: 850-414-6122 Email: Donna.Smith@dms.myflorida.com For issues with MyFloridaMarketPlace (MFMP), please call the Customer Service Desk at 1-866352-3776 or e-mail, VendorHelp@myfloridamarketplace.com 3.2 Definitions The definitions found in s. 60A-1.001, F.A.C. shall apply to this agreement. In addition to definitions found in Section 2.1, the following terms are also defined: a. "The Department" means the Department of Management Services. b. “Eligible Users” is defined in 60A-1.005, F.A.C. The following entities are eligible users: 1. All governmental agencies, as defined in Section 163.3164, F.S., which have a physical presence within the State of Florida; 2. Any independent, non-profit college or university that is located within the State of Florida and is accredited by the Southern Association of Colleges and Schools. c. “Contractor” means any person who contracts to sell commodities or contractual services to an agency. d. “Customer” means any agency or eligible user placing an order through a contract resulting from this solicitation. 3.3 Firm Response The Department may make an award within one-hundred and eighty (180) days after the date of the opening, during which period responses shall remain firm and shall not be withdrawn. If award is not made within one-hundred and eighty (180) days, the response shall remain firm until either the Department awards the contract or the Department receives from the Respondent written notice that the response is withdrawn. Any response that expresses a shorter duration may, in the Department’s sole discretion, be accepted or rejected. RFP No. 10-475-000-J Medical and Dental Supplies Page 13 3.4 Electronic Posting of Award Offers shall be opened on the date and time indicated on the Timeline and thereafter evaluated. Prices will not be read, pursuant to Section 119.07(3) (m) of the Florida Statutes. After evaluating the responses, the Department shall electronically post a Notice of Intent to Award at http://fcn.state.fl.us/owa_vbs/owa/vbs_www.main_menu Respondents are encouraged to check the Vendor Bid System (VBS) (see link above) regularly for postings. The VBS does not release an e-mail for Agency Decisions. Any person who is adversely affected by the decision shall file with the Department a notice of protest within 72 hours after the electronic posting (see Section 2.19 of the General Instructions (PUR 1001) for more information on protests. The Department shall not provide notices of award by telephone. 3.5 Order of Precedence Potential Respondents to the solicitation are encouraged to carefully review all the materials contained herein and prepare responses accordingly. In the event any conflict exists between the Special and General Instructions, those instructions specified in the Special Instructions to Respondents shall prevail. After successful evaluations, Contractor(s) shall sign a Contract form incorporating the solicitation materials and any additional terms and conditions resulting from the negotiation process. All responses are subject to the terms of the following sections of this solicitation, which, in case of conflict, shall have the order of precedence listed: Section 1.0, Introduction Section 6.0, Technical Specifications Section 5.0, Special Contract Conditions, Section 4.0, General Contract Conditions (PUR 1000) Section 3.0, Special Instructions to Respondents Section 2.0, General Instructions to Respondents (PUR 1001) Section 7.0, Forms and Price Sheets Reminder: Above order takes precedence over order listed in Section 2.4. 3.6 Submittal of Response Respondents shall submit their offer by the deadline on one CD and include 2 identical copies on individual CDs (a total of 3 CDs), sent or delivered in a sealed envelope to the Contact listed in Section 3.1. The solicitation number should be clearly marked on the outside of the envelope or package. Label the CDs with your company’s name and the solicitation number, 10-475-000J. Check your CDs to make sure they can be read by any computer (Windows® operating system). All forms shall be completed using Microsoft Word® or equivalent for legibility. The Excel® spreadsheets are pre loaded with a formula. Download the spreadsheets from MFMP – the price sheets contained in this document do not have the formula loaded and are for reference only. Enter the list price of each item you are bidding on, then the discount you are offering and “Price to the State” will be calculated for you. RFP No. 10-475-000-J Medical and Dental Supplies Page 14 You are not required to bid on every item, but leave the entire spreadsheet intact (do not delete lines you are not bidding on.) Altered spreadsheets cannot be scored. For this solicitation only, responses will NOT be accepted on-line via the MyFloridaMarketPlace sourcing tool located at https://sourcing.myfloridamarketplace.com. This section negates the electronic response requirement in Section 2.3. Each Respondent is responsible for ensuring that its response and all associated documents are submitted by the proper date and time. Dun and Bradstreet reports must also be delivered to the address noted in Section 3.1 if they are not included on the response CD. Failure to provide all requested information within the response package by the date and time specified may result in rejection of the response. The Proposal Preparation Checklist included within this solicitation does not relieve the Respondent of responsibility for ensuring that all requirements of the solicitation are included with the submittal. DO NOT RELY ON THE MYFLORIDAMARKETPLACE SOURCING TOOL’S TIME REMAINING CLOCK. THE OFFICIAL SOLICITATION CLOSING TIME SHALL BE AS REFLECTED IN SECTION 1.2, TIMELINE. The response deadline(s) shall be as reflected in Section 1.2, Timeline, of this solicitation. The MyFloridaMarketPlace Sourcing Tool’s time remaining clock is not the official submission date and time deadline, it is intended only to approximate the solicitation closing and may require periodic adjustments 3.7 Addendums to the Solicitation Documents The Department reserves the right to issue addendums to the solicitation. Notice of any addendum will be posted within MyFloridaMarketPlace and the Vendor Bid System. Such notice, if required, will contain the appropriate details for identifying and/or reviewing the formal changes to the solicitation. Each Respondent is responsible for monitoring the sites for new or changing information concerning this solicitation. 3.8 Submittal Content The Department requires that Respondents follow the response format outlined below: File A: Qualifications Profile: Describe your organization and general background. Include information on your organization’s size, recent sales history, which manufacturers have authorized your company as a dealer, service philosophy and complaint resolution process. Explain your Vendor Managed Inventory (VMI) capabilities. History and Experience: Provide years of experience in the medical and or dental supply manufacturer or distribution industry (nationally and/or in Florida). Provide number of years servicing the public sector. Identify key management personnel and any individuals to be assigned to this contract. RFP No. 10-475-000-J Medical and Dental Supplies Page 15 Litigation: List any litigation or disputes relating to the required services within the last five years. Include entity/project name, timeframe, and the nature of litigation. Specifically identify any pending or active litigation. Financial Status: Each Respondent is required to provide information regarding its financial and industry standing and strength in order to demonstrate that it is financially stable, in good standing with creditors, and has the resources necessary to perform the services outlined in this RFP on a state-wide and/or regional basis. The State requires each Respondent to provide a Supplier Qualifier Report (SQR) prepared by Dun & Bradstreet (D&B), in accordance with the instructions below. The SQR is a standard report that details financial and operational capabilities. This report must be submitted to the Department either as a file on the submitted CDs or delivered prior to the bid opening date and time (See Section 1.2 Event Timeline.) Each Respondent will be responsible for the cost and timely submission of this report. The prospective Contractor will request the SQR from D&B at: https://sor.dnb.com/sor/jsp/forms/SOF.jsp?SORTAG1=JQ37hS4r&SORTAG2=j58Gjk4x 1. Enter the RFP number in the text field entitled “Enter your RFP Number” and select “Submit.” 2. Enter your company’s Dun Number. (If you don’t know your company’s Dun number, you may use the search feature to find it.) 3. 4. Confirm Registration Enter payment method and information and complete registration. The cost of the preparation of the D&B report shall be the responsibility of the Respondent. If your company is not eligible for a Dun and Bradstreet report (privately held), please include financial statements for the last three years, in accordance with Generally Accepted Accounting Principles. File B: Reference Summaries, Attachment 2 Provide a minimum of five references, large governmental and/or commercial accounts preferred. Information shall include: o Client name, address, phone number, fax number and email address. File C: Experience Certification Form, Attachment 3 Certifies a minimum of 5 years of experience in providing medical and dental supplies. Requires a notary seal. If not applicable to your company, please include that statement instead of the form. File D: Respondent Capability Form, Attachment 4 This form identifies your capabilities to service certain locations Include a copy of your company’s Liability Insurance policy, declaration page only. File E: Ordering Instructions Form, Attachment 5 RFP No. 10-475-000-J Medical and Dental Supplies Page 16 File F: Certification of Drug Free Workplace Program, Attachment 6 File G Price Sheets, Attachment 7 The Price Sheets are Excel ® spreadsheets and list Core Items. The Non Core items are not listed. Please offer a standard discount off MSRP for ALL Non Core items. This standard discount is for any item not on the Core Item List - See Section 6.3 Scope. 3.9 Estimated Sales State agencies have spent $23,109,159 on the commodity codes listed in Section 1.1 between January 1, 2008 and November 5, 2009. Respondents are reminded that these figures do not represent any guarantee on the number of orders or continued spend by the agencies or eligible users. Other eligible users’ spend is not included in this number. This figure is given only as a guideline for preparing your response and should not be construed as representing actual commitment figures under the contract. The historical spend data, gathered from 17 state agencies is as follows and again does not guarantee any level of business to the awarded supplier(s); 2008 Agency for Health Care Administration $244.55 2009 Agency for Health Care Administration $16.52 2008 Agency for Persons with Disabilities $233,764.94 2009 Agency for Persons with Disabilities $163,883.47 2008 Department of Children and Families $170,963.74 2009 Department of Children and Families $38,263.41 2008 Department of Community Affairs $60,208.07 2009 Department of Community Affairs $40.68 2008 Department of Corrections $4,892,597.27 2009 Department of Corrections $1,810,788.33 2008 Department of Education $855.14 2009 Department of Education $733.20 2008 Department of Environmental Protection $39,167.44 2009 Department of Environmental Protection $8,096.64 2008 Department of Financial Services $2,422.17 2009 Department of Financial Services $0.00 2008 Department of Health $6,251,929.83 2009 Department of Health $8,446,395.73 2008 Department of Highway Safety and Motor Vehicles $46,595.00 2009 Department of Highway Safety and Motor Vehicles $15,649.15 2008 Department of Juvenile Justice $118,405.45 2009 Department of Juvenile Justice $87,510.45 2008 Department of Law Enforcement $23,611.15 2009 Department of Law Enforcement $20,923.99 RFP No. 10-475-000-J Medical and Dental Supplies Page 17 2008 Department of Veterans Affairs $487.00 2009 Department of Veterans Affairs $663,751.08 2008 Executive Office of the Governor $196.89 2009 Executive Office of the Governor $3.90 2008 Fish and Wildlife Conservation Commission $1,295.00 2008 Florida School for the Deaf and the Blind $3,309.20 2009 Florida School for the Deaf and the Blind $2,232.51 2008 State Courts System $28.65 2009 State Courts System $4,789.04 Total 3.10 $23,109,159.59 Evaluation and Selection Process The Department shall evaluate and rank responsive replies against all evaluation criteria set forth in the solicitation. The Department shall award a contract to the responsive and responsible Respondent(s) that the Department determines will provide the best value to the State and whose response earned a minimum of 70 points. The Department reserves the right to reject any and all replies, if the Department determines such action is in the best interest of the State. Responses will be scored on a scale of 1 to 100 using the following criteria: Qualifications: Price Total 40% 60% 100% 40 points 60 points 100 points There will be three Evaluators. These Evaluators will rank the Responses independently and their scores will be averaged to obtain a Respondents actual score per category and in total. Qualifications: Measuring the Respondent’s qualifications, capability, and experience in successfully serving facilities of similar size and scope to those required by this solicitation Maximum 40 points. A minimum of 30 points must be achieved in the Qualifications section to be eligible for final award. General Capabilities: maximum 20 points o o o o Consider responses in File A Licenses, certifications, and insurance should be current. Consider size of company, locations and extent of company’s services to potential contract needs. Consider Respondents Vendor Managed Inventory (VMI) capabilities. Financial Status: maximum 10 points o o Consider responses in File A. Historical sales, accounting reports and identifiable trends should identify strong financial practices. RFP No. 10-475-000-J Medical and Dental Supplies Page 18 o Recent financial report (Dun & Bradstreet or equivalent) should identify short-term viability. Experience and References: maximum 10 points o o o Preference for companies with more than 5 years experience producing and or distributing quality medical and dental supplies. Preference for companies that have considerable and satisfactory experience providing services to governmental entities. Preference for companies that have a proven ability to effectively service and manage multiple sites in a regional area. Price: 60 Maximum points are available in this category. Respondent’s price will be compared against other responses and against national industry averages. 3.11 Consider Respondent’s pricing in comparison with other Respondents. Final prices on Core items are more important than the discount percentage. Basis for Award Award(s) may be made by district. The Department shall award a contract to the responsive and responsible Respondent(s) that the Department determines will provide the best value to the State and whose response earned a minimum of 70 points (minimum 30 points in qualifications). The Department reserves the right to reject any and all replies, if the Department determines such action is in the best interest of the State. The Department further reserves the right to award the contract, if any, to a single Respondent. 3.12 Price Pricing on any state term contract resulting from this solicitation will be an established ceiling price. Customers are encouraged to seek quotes from multiple contractors and to negotiate lower costs in accordance with their particular project or needs. . 3.13 Lobbying Reference contract section 2.21 Limitation on Vendor Contact with Agency during Solicitation Period. Respondents are advised that the following will be included in the Contract for these services: In accordance with Section 216.347, Florida Statutes, and as provided herein, the Service Provider or Contractor may not expend any State funds for the purpose of lobbying the legislature, the judicial branch, the executive branch, or any State Agency. 3.14 Documentation Becomes the Property of the State RFP No. 10-475-000-J Medical and Dental Supplies Page 19 All documentation produced as part of this solicitation shall become the exclusive property of the State. Documentation may not be removed by the Respondent or its agents and will not be returned to the Respondent. Selection or rejection of a reply shall not affect this right. 3.15 Notice to Contractor The employment of unauthorized aliens by any contractor is considered a violation of section 247A (e) of the Immigration and Nationalization Act. If the contractor knowingly employs unauthorized aliens, such violation shall be cause for unilateral cancellation of the contract. 3.16 Method of Payment The State of Florida has implemented a purchasing card program, using the Visa platform. Contractors may receive payment from state agencies by the purchasing card in the same manner as other Visa purchases. Visa acceptance is mandatory but is not the exclusive method of payment. The State will not fill out any contractor forms or contracts in association with the Contractor accepting a purchasing card payment. Contractors are not allowed to charge a fee for accepting a purchasing card payment. Surcharges or convenience fees are prohibited. Fees should not be charged for using a purchasing card unless the fees are charged for all methods of payment (cash, check, debit cards, vouchers, etc.), and must be approved by the Customer prior to order acceptance. On-line billing or payment systems maintained by the Contractor will not store the card holder’s account number and expiration date for re-use. Card holders will provide the Contractor with card account information at each transaction. 3.17 Environmental Considerations The State supports and encourages initiatives to protect and preserve our environment. The Respondent shall submit as part of any proposal the Respondent’s plan to support the procurement of products and materials with recycled content, and the intent of Section 287.045, Florida Statutes. The Respondent shall also provide a plan for reducing and or handling of any hazardous waste generated by Respondent’s company. Reference Rule 62-730.160, Florida Administrative Code. It is a requirement of the Florida Department of Environmental Protection that a generator of hazardous waste materials that exceeds a certain threshold must have a valid and current Hazardous Waste Generator Identification Number. This identification number shall be submitted as part of Respondent’s explanation of its company’s hazardous waste plan and shall explain in detail its handling and disposal of this waste. Respondents are asked to identify environmentally friendly products and the Standard by which that product is rated. 3.18 Certification of Drug-Free Workplace Program The State supports and encourages initiatives to keep the workplaces of Florida’s contractors and businesses drug free. Section 287.087 of the Florida Statutes provides that, where identical tie Responses are received, preference shall be given to a proposal received from a Respondent that certifies it has implemented a drug-free workforce program. RFP No. 10-475-000-J Medical and Dental Supplies Page 20 3.19 Public Entity Crimes A person or affiliate who has been placed on the convicted vendor list following a conviction for a public entity crime may not submit a response on a request to provide any goods or services to a public entity, may not submit a response on a contract with a public entity for the construction or repair of a public building or public work, may not submit responses on leases of real property to a public entity, may not be awarded or perform work as a contractor, supplier, subcontractor, or consultant under a contract with any public entity, and may not transact business with any public entity in excess of the threshold amount provided in F.S. 287.017 for CATEGORY TWO for a period of thirty-six (36) months from the date of being placed on the convicted vendor list. The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 21 SECTION 4 GENERAL CONTRACT CONDITIONS (STATE OF FLORIDA FORM PUR 1000) 4.1 Definitions. The definitions contained in s. 60A-1.001, F.A.C. shall apply to this agreement. The following additional terms are also defined: (a) “Contract” means the legally enforceable agreement that results from a successful solicitation. The parties to the Contract will be the Customer and Contractor. (b) “Customer” means the State agency or other entity identified in a contract as the party to receive commodities or contractual services pursuant to a contract or that orders commodities or contractual services via purchase order or other contractual instrument from the Contractor under the Contract. The “Customer” may also be the “Buyer” as defined in the PUR 1001 if it meets the definition of both terms. (c) “Product” means any deliverable under the Contract, which may include commodities, services, technology or software. (d) “Purchase order” means the form or format a Customer uses to make a purchase under the Contract (e.g., a formal written purchase order, electronic purchase order, procurement card, contract or other authorized means). 4.2 Purchase Orders. In contracts where commodities or services are ordered by the Customer via purchase order, Contractor shall not deliver or furnish products until a Customer transmits a purchase order. All purchase orders shall bear the Contract or solicitation number, shall be placed by the Customer directly with the Contractor, and shall be deemed to incorporate by reference the Contract and solicitation terms and conditions. Any discrepancy between the Contract terms and the terms stated on the Contractor’s order form, confirmation, or acknowledgement shall be resolved in favor of terms most favorable to the Customer. A purchase order for services within the ambit of section 287.058(1) of the Florida Statutes shall be deemed to incorporate by reference the requirements of subparagraphs (a) through (f) thereof. Customers shall designate a contract manager and a contract administrator as required by subsections 287.057(15) and (16) of the Florida Statutes. 4.3 Product Version. Purchase orders shall be deemed to reference a manufacturer’s most recently release model or version of the product at the time of the order, unless the Customer specifically requests in writing an earlier model or version and the contractor is willing to provide such model or version. 4.4 Price Changes Applicable only to Term Contracts. If this is a term contract for commodities or services, the following provisions apply. (a) Quantity Discounts. Contractors are urged to offer additional discounts for one time delivery of large single orders. Customers should seek to negotiate additional price concessions on quantity purchases of any products offered under the Contract. State Customers shall document their files accordingly. (b) Best Pricing Offer. During the Contract term, if the Customer becomes aware of better pricing offered by the Contractor for substantially the same or a smaller quantity of a product RFP No. 10-475-000-J Medical and Dental Supplies Page 22 outside the Contract, but upon the same or similar terms of the Contract, then at the discretion of the Customer the price under the Contract shall be immediately reduced to the lower price. (c) Sales Promotions. In addition to decreasing prices for the balance of the Contract term due to a change in market conditions, a Contractor may conduct sales promotions involving price reductions for a specified lesser period. A Contractor shall submit to the Contract Specialist documentation identifying the proposed (1) starting and ending dates of the promotion, (2) products involved, and (3) promotional prices compared to then-authorized prices. Promotional prices shall be available to all Customers. Upon approval, the Contractor shall provide conspicuous notice of the promotion. (d) Trade-In. Customers may trade-in equipment when making purchases from the Contract. A trade-in shall be negotiated between the Customer and the Contractor. Customers are obligated to actively seek current fair market value when trading equipment, and to keep accurate records of the process. For State agencies, it may be necessary to provide documentation to the Department of Financial Services and to the agency property custodian pursuant to Chapter 273, F.S. (e) Equitable Adjustment. The Customer may, in its sole discretion, make an equitable adjustment in the Contract terms or pricing if pricing or availability of supply is affected by extreme and unforeseen volatility in the marketplace, that is, by circumstances that satisfy all the following criteria: (1) the volatility is due to causes wholly beyond the Contractor’s control, (2) the volatility affects the marketplace or industry, not just the particular Contract source of supply, (3) the effect on pricing or availability of supply is substantial, and (4) the volatility so affects the Contractor that continued performance of the Contract would result in a substantial loss. 4.5 Additional Quantities. For a period not exceeding ninety (90) days from the date of solicitation award, the Customer reserves the right to acquire additional quantities up to the amount shown on the solicitation but not to exceed the threshold for Category Two at the prices submitted in the response to the solicitation. 4.6 Packaging. Tangible product shall be securely and properly packed for shipment, storage, and stocking in appropriate, clearly labeled, shipping containers and according to accepted commercial practice, without extra charge for packing materials, cases, or other types of containers. All containers and packaging shall become and remain Customer’s property. 4.7 Inspection at Contractor’s Site. The Customer reserves the right to inspect, at any reasonable time with prior notice, the equipment or product or plant or other facilities of a Contractor to assess conformity with Contract requirements and to determine whether they are adequate and suitable for proper and effective Contract performance. 4.8 Safety Standards. All manufactured items and fabricated assemblies subject to operation under pressure, operation by connection to an electric source, or operation involving connection to a manufactured, natural, or LP gas source shall be constructed and approved in a manner acceptable to the appropriate State inspector. Acceptability customarily requires, at a minimum, identification marking of the appropriate safety standard organization, where such approvals of listings have been established for the type of device offered and furnished, for example: the American Society of Mechanical Engineers for pressure vessels; the Underwriters Laboratories RFP No. 10-475-000-J Medical and Dental Supplies Page 23 and/or National Electrical Manufacturers’ Association for electrically operated assemblies; and the American Gas Association for gas-operated assemblies. In addition, all items furnished shall meet all applicable requirements of the Occupational Safety and Health Act and state and federal requirements relating to clean air and water pollution. 4.9 Americans with Disabilities Act. Contractors should identify any products that may be used or adapted for use by visually, hearing, or other physically impaired individuals. 4.10 Literature. Upon request, the Contractor shall furnish literature reasonably related to the product offered, for example, user manuals, price schedules, catalogs, descriptive brochures, etc. 4.11 Transportation and Delivery. Prices shall include all charges for packing, handling, freight, distribution, and inside delivery. Transportation of goods shall be FOB Destination to any point within thirty (30) days after the Customer places an Order. A Contractor, within five (5) days after receiving a purchase order, shall notify the Customer of any potential delivery delays. Evidence of inability or intentional delays shall be cause for Contract cancellation and Contractor suspension. 4.12 Installation. Where installation is required, Contractor shall be responsible for placing and installing the product in the required locations at no additional charge, unless otherwise designated on the Contract or purchase order. Contractor’s authorized product and price list shall clearly and separately identify any additional installation charges. All materials used in the installation shall be of good quality and shall be free of defects that would diminish the appearance of the product or render it structurally or operationally unsound. Installation includes the furnishing of any equipment, rigging, and materials required to install or replace the product in the proper location. Contractor shall protect the site from damage and shall repair damages or injury caused during installation by Contractor or its employees or agents. If any alteration, dismantling, excavation, etc., is required to achieve installation, the Contractor shall promptly restore the structure or site to its original condition. Contractor shall perform installation work so as to cause the least inconvenience and interference with Customers and with proper consideration of others on site. Upon completion of the installation, the location and surrounding area of work shall be left clean and in a neat and unobstructed condition, with everything in satisfactory repair and order. 4.13 Risk of Loss. Matters of inspection and acceptance are addressed in s. 215.422, F.S. Until acceptance, risk of loss or damage shall remain with the Contractor. The Contractor shall be responsible for filing, processing, and collecting all damage claims. To assist the Contractor with damage claims, the Customer shall: record any evidence of visible damage on all copies of the delivering carrier’s Bill of Lading; report damages to the carrier and the Contractor; and provide the Contractor with a copy of the carrier’s Bill of Lading and damage inspection report. When a Customer rejects a product, Contractor shall remove it from the premises within ten days after notification or rejection. Upon rejection notification, the risk of loss of rejected or nonconforming product shall remain with the Contractor. Rejected product not removed by the Contractor within ten days shall be deemed abandoned by the Contractor, and the Customer shall have the right to dispose of it as its own property. Contractor shall reimburse the Customer for costs and expenses incurred in storing or effecting removal or disposition of rejected product. 4.14 Transaction Fee. The State of Florida has instituted MyFloridaMarketPlace, a statewide eProcurement System (“System”). Pursuant to section 287.057(23), Florida Statutes (2002), all RFP No. 10-475-000-J Medical and Dental Supplies Page 24 payments shall be assessed a Transaction Fee of one percent (1.0%), which the Contractor shall pay to the State, unless exempt pursuant to 60A-1.032, F.A.C. For payments within the State accounting system (FLAIR or its successor), the Transaction Fee shall, when possible, be automatically deducted from payments to the Contractor. If automatic deduction is not possible, the Contractor shall pay the Transaction Fee pursuant to Rule 60A1.031(2), F.A.C. By submission of these reports and corresponding payments, Contractor certifies their correctness. All such reports and payments shall be subject to audit by the State or its designee. Contractor shall receive a credit for any Transaction Fee paid by the Contractor for the purchase of any item(s) if such item(s) are returned to the Contractor through no fault, act, or omission of the Contractor. Notwithstanding the foregoing, a Transaction Fee is non-refundable when an item is rejected or returned, or declined, due to the Contractor’s failure to perform or comply with specifications or requirements of the agreement. Failure to comply with these requirements shall constitute grounds for declaring the Contractor in default and recovering reprocurement costs from the Contractor in addition to all outstanding fees. CONTRACTORS DELINQUENT IN PAYING TRANSACTION FEES MAY BE SUBJECT TO BEING REMOVED FROM THE DEPARTMENT OF MANAGEMENT SERVICES’ VENDOR LIST AS PROVIDED IN RULE 60A-1.006, F.A.C. 4.15 Invoicing and Payment. Invoices shall contain the Contract number, purchase order number if applicable, and the appropriate vendor identification number. The State may require any other information from the Contractor that the State deems necessary to verify any purchase order placed under the Contract. At the State's option, Contractors may be required to invoice electronically pursuant to guidelines of the Department of Management Services. Current guidelines require that Contractor supply electronic invoices in lieu of paper-based invoices for those transactions processed through the system. Electronic invoices shall be submitted to the Customer through the Ariba Supplier Network (ASN) in one of the following mechanisms – EDI 810, cXML, or webbased invoice entry within the ASN. Payment shall be made in accordance with sections 215.422 and 287.0585 of the Florida Statutes, which govern time limits for payment of invoices. Invoices that must be returned to a Contractor due to preparation errors will result in a delay in payment. Contractors may call (850) 413-7269 Monday through Friday to inquire about the status of payments by State Agencies. The Customer is responsible for all payments under the Contract. A Customer’s failure to pay, or delay in payment, shall not constitute a breach of the Contract and shall not relieve the Contractor of its obligations to the Department or to other Customers. 4.16 Taxes. The State does not pay Federal excise or sales taxes on direct purchases of tangible personal property. The State will not pay for any personal property taxes levied on the Contractor or for any taxes levied on employees’ wages. Any exceptions to this paragraph shall be explicitly noted by the Customer in the special contract conditions section of the solicitation or in the Contract or purchase order. 4.17 Governmental Restrictions. If the Contractor believes that any governmental restrictions have been imposed that require alteration of the material, quality, workmanship or performance of the products offered under the Contract, the Contractor shall immediately notify the Customer in writing, indicating the specific restriction. The Customer reserves the right and the complete discretion to accept any such alteration or to cancel the Contract at no further expense to the Customer. RFP No. 10-475-000-J Medical and Dental Supplies Page 25 4.18 Lobbying and Integrity. Customers shall ensure compliance with Section 11.062, FS and Section 216.347, F.S. The Contractor shall not, in connection with this or any other agreement with the State, directly or indirectly (1) offer, confer, or agree to confer any pecuniary benefit on anyone as consideration for any State officer or employee’s decision, opinion, recommendation, vote, other exercise of discretion, or violation of a known legal duty, or (2) offer, give, or agree to give to anyone any gratuity for the benefit of, or at the direction or request of, any State officer or employee. For purposes of clause (2), “gratuity” means any payment of more than nominal monetary value in the form of cash, travel, entertainment, gifts, meals, lodging, loans, subscriptions, advances, deposits of money, services, employment, or contracts of any kind. Upon request of the Customer’s Inspector General, or other authorized State official, the Contractor shall provide any type of information the Inspector General deems relevant to the Contractor’s integrity or responsibility. Such information may include, but shall not be limited to, the Contractor’s business or financial records, documents, or files of any type or form that refer to or relate to the Contract. The Contractor shall retain such records for the longer of (1) three years after the expiration of the Contract or (2) the period required by the General Records Schedules maintained by the Florida Department of State (available at: http://dlis.dos.state.fl.us/barm/genschedules/gensched.htm). The Contractor agrees to reimburse the State for the reasonable costs of investigation incurred by the Inspector General or other authorized State official for investigations of the Contractor’s compliance with the terms of this or any other agreement between the Contractor and the State which results in the suspension or debarment of the Contractor. Such costs shall include, but shall not be limited to: salaries of investigators, including overtime; travel and lodging expenses; and expert witness and documentary fees. The Contractor shall not be responsible for any costs of investigations that do not result in the Contractor’s suspension or debarment. 4.19 Indemnification. The Contractor shall be fully liable for the actions of its agents, employees, partners, or subcontractors and shall fully indemnify, defend, and hold harmless the State and Customers, and their officers, agents, and employees, from suits, actions, damages, and costs of every name and description, including attorneys’ fees, arising from or relating to personal injury and damage to real or personal tangible property alleged to be caused in whole or in part by Contractor, its agents, employees, partners, or subcontractors, provided, however, that the Contractor shall not indemnify for that portion of any loss or damages proximately caused by the negligent act or omission of the State or a Customer. Further, the Contractor shall fully indemnify, defend, and hold harmless the State and Customers from any suits, actions, damages, and costs of every name and description, including attorneys’ fees, arising from or relating to violation or infringement of a trademark, copyright, patent, trade secret or intellectual property right, provided, however, that the foregoing obligation shall not apply to a Customer’s misuse or modification of Contractor’s products or a Customer’s operation or use of Contractor’s products in a manner not contemplated by the Contract or the purchase order. If any product is the subject of an infringement suit or in the Contractor’s opinion is likely to become the subject of such a suit, the Contractor may at its sole expense procure for the Customer the right to continue using the product or to modify it to become non-infringing. If the Contractor is not reasonably able to modify or otherwise secure the Customer the right to continue using the product, the Contractor shall remove the product and refund the Customer the amounts paid in excess of a reasonable rental for past use. The customer shall not be liable for any royalties. RFP No. 10-475-000-J Medical and Dental Supplies Page 26 The Contractor’s obligations under the preceding two paragraphs with respect to any legal action are contingent upon the State or Customer giving the Contractor (1) written notice of any action or threatened action, (2) the opportunity to take over and settle or defend any such action at Contractor’s sole expense, and (3) assistance in defending the action at Contractor’s sole expense. The Contractor shall not be liable for any cost, expense, or compromise incurred or made by the State or Customer in any legal action without the Contractor’s prior written consent, which shall not be unreasonably withheld. 4.20 Limitation of Liability. For all claims against the Contractor under any contract or purchase order, and regardless of the basis on which the claim is made, the Contractor’s liability under a contract or purchase order for direct damages shall be limited to the greater of $100,000, the dollar amount of the contract or purchase order, or two times the charges rendered by the Contractor under the purchase order. This limitation shall not apply to claims arising under the Indemnity paragraph contain in this agreement. Unless otherwise specifically enumerated in the Contract or in the purchase order, no party shall be liable to another for special, indirect, punitive, or consequential damages, including lost data or records (unless the contract or purchase order requires the Contractor to back-up data or records), even if the party has been advised that such damages are possible. No party shall be liable for lost profits, lost revenue, or lost institutional operating savings. The State and Customer may, in addition to other remedies available to them at law or equity and upon notice to the Contractor, retain such monies from amounts due Contractor as may be necessary to satisfy any claim for damages, penalties, costs and the like asserted by or against them. The State may set off any liability or other obligation of the Contractor or its affiliates to the State against any payments due the Contractor under any contract with the State. 4.21 Suspension of Work. The Customer may in its sole discretion suspend any or all activities under the Contract or purchase order, at any time, when in the best interests of the State to do so. The Customer shall provide the Contractor written notice outlining the particulars of suspension. Examples of the reason for suspension include, but are not limited to, budgetary constraints, declaration of emergency, or other such circumstances. After receiving a suspension notice, the Contractor shall comply with the notice and shall not accept any purchase orders. Within ninety days, or any longer period agreed to by the Contractor, the Customer shall either (1) issue a notice authorizing resumption of work, at which time activity shall resume, or (2) terminate the Contract or purchase order. Suspension of work shall not entitle the Contractor to any additional compensation. 4.22 Termination for Convenience. The Customer, by written notice to the Contractor, may terminate the Contract in whole or in part when the Customer determines in its sole discretion that it is in the State’s interest to do so. The Contractor shall not furnish any product after it receives the notice of termination, except as necessary to complete the continued portion of the Contract, if any. The Contractor shall not be entitled to recover any cancellation charges or lost profits. 4.23 Termination for Cause. The Customer may terminate the Contract if the Contractor fails to (1) deliver the product within the time specified in the Contract or any extension, (2) maintain adequate progress, thus endangering performance of the Contract, (3) honor any term of the Contract, or (4) abide by any statutory, regulatory, or licensing requirement. Rule 60A-1.006(3), F.A.C., governs the procedure and consequences of default. The Contractor shall continue work on any work not terminated. Except for defaults of subcontractors at any tier, the Contractor shall not be liable for any excess costs if the failure to perform the Contract arises RFP No. 10-475-000-J Medical and Dental Supplies Page 27 from events completely beyond the control, and without the fault or negligence, of the Contractor. If the failure to perform is caused by the default of a subcontractor at any tier, and if the cause of the default is completely beyond the control of both the Contractor and the subcontractor, and without the fault or negligence of either, the Contractor shall not be liable for any excess costs for failure to perform, unless the subcontracted products were obtainable from other sources in sufficient time for the Contractor to meet the required delivery schedule. If, after termination, it is determined that the Contractor was not in default, or that the default was excusable, the rights and obligations of the parties shall be the same as if the termination had been issued for the convenience of the Customer. The rights and remedies of the Customer in this clause are in addition to any other rights and remedies provided by law or under the Contract. 4.24 Force Majeure, Notice of Delay, and No Damages for Delay. The Contractor shall not be responsible for delay resulting from its failure to perform if neither the fault nor the negligence of the Contractor or its employees or agents contributed to the delay and the delay is due directly to acts of God, wars, acts of public enemies, strikes, fires, floods, or other similar cause wholly beyond the Contractor’s control, or for any of the foregoing that affect subcontractors or suppliers if no alternate source of supply is available to the Contractor. In case of any delay the Contractor believes is excusable, the Contractor shall notify the Customer in writing of the delay or potential delay and describe the cause of the delay either (1) within ten (10) days after the cause that creates or will create the delay first arose, if the Contractor could reasonably foresee that a delay could occur as a result, or (2) if delay is not reasonably foreseeable, within five (5) days after the date the Contractor first had reason to believe that a delay could result. THE FOREGOING SHALL CONSTITUTE THE CONTRACTOR’S SOLE REMEDY OR EXCUSE WITH RESPECT TO DELAY. Providing notice in strict accordance with this paragraph is a condition precedent to such remedy. No claim for damages, other than for an extension of time, shall be asserted against the Customer. The Contractor shall not be entitled to an increase in the Contract price or payment of any kind from the Customer for direct, indirect, consequential, impact or other costs, expenses or damages, including but not limited to costs of acceleration or inefficiency, arising because of delay, disruption, interference, or hindrance from any cause whatsoever. If performance is suspended or delayed, in whole or in part, due to any of the causes described in this paragraph, after the causes have ceased to exist the Contractor shall perform at no increased cost, unless the Customer determines, in its sole discretion, that the delay will significantly impair the value of the Contract to the State or to Customers, in which case the Customer may (1) accept allocated performance or deliveries from the Contractor, provided that the Contractor grants preferential treatment to Customers with respect to products subjected to allocation, or (2) purchase from other sources (without recourse to and by the Contractor for the related costs and expenses) to replace all or part of the products that are the subject of the delay, which purchases may be deducted from the Contract quantity, or (3) terminate the Contract in whole or in part. 4.25 Changes. The Customer may unilaterally require, by written order, changes altering, adding to, or deducting from the Contract specifications, provided that such changes are within the general scope of the Contract. The Customer may make an equitable adjustment in the Contract price or delivery date if the change affects the cost or time of performance. Such equitable adjustments require the written consent of the Contractor, which shall not be unreasonably withheld. If unusual quantity requirements arise, the Customer may solicit separate bids to satisfy them. 4.26 Renewal. Upon mutual agreement, the Customer and the Contractor may renew the Contract, in whole or in part, for a period that may not exceed 3 years or the term of the RFP No. 10-475-000-J Medical and Dental Supplies Page 28 contract, whichever period is longer. Any renewal shall specify the renewal price, as set forth in the solicitation response. The renewal must be in writing and signed by both parties, and is contingent upon satisfactory performance evaluations and subject to availability of funds. 4.27 Purchase Order Duration. Purchase orders issued pursuant to a state term or agency contract must be received by the Contractor no later than close of business on the last day of the contract’s term to be considered timely. The Contractor is obliged to fill those orders in accordance with the contract’s terms and conditions. Purchase orders received by the contractor after close of business on the last day of the state term or agency contract’s term shall be considered void. Purchase orders for a one-time delivery of commodities or performance of contractual services shall be valid through the performance by the Contractor, and all terms and conditions of the state term or agency contract shall apply to the single delivery/performance, and shall survive the termination of the Contract. Contractors are required to accept purchase orders specifying delivery schedules exceeding the contracted schedule even when such extended delivery will occur after expiration of the state term or agency contract. For example, if a state term contract calls for delivery 30 days after receipt of order (ARO), and an order specifies delivery will occur both in excess of 30 days ARO and after expiration of the state term contract, the Contractor will accept the order. However, if the Contractor expressly and in writing notifies the ordering office within ten (10) calendar days of receipt of the purchase order that Contractor will not accept the extended delivery terms beyond the expiration of the state term contract, then the purchase order will either be amended in writing by the ordering entity within ten (10) calendar days of receipt of the contractor’s notice to reflect the state term contract delivery schedule, or it shall be considered withdrawn. The duration of purchase orders for recurring deliveries of commodities or performance of services shall not exceed the expiration of the state term or agency contract by more than twelve months. However, if an extended pricing plan offered in the state term or agency contract is selected by the ordering entity, the contract terms on pricing plans and renewals shall govern the maximum duration of purchase orders reflecting such pricing plans and renewals. Timely purchase orders shall be valid through their specified term and performance by the Contractor, and all terms and conditions of the state term or agency contract shall apply to the recurring delivery/performance as provided herein, and shall survive the termination of the Contract. Ordering offices shall not renew a purchase order issued pursuant to a state term or agency contract if the underlying contract expires prior to the effective date of the renewal. 4.28 Advertising. Subject to Chapter 119, Florida Statutes, the Contractor shall not publicly disseminate any information concerning the Contract without prior written approval from the Customer, including, but not limited to mentioning the Contract in a press release or other promotional material, identifying the Customer or the State as a reference, or otherwise linking the Contractor’s name and either a description of the Contract or the name of the State or the Customer in any material published, either in print or electronically, to any entity that is not a party to Contract, except potential or actual authorized distributors, dealers, resellers, or service representative. RFP No. 10-475-000-J Medical and Dental Supplies Page 29 4.29 Assignment. The Contractor shall not sell, assign or transfer any of its rights, duties or obligations under the Contract, or under any purchase order issued pursuant to the Contract, without the prior written consent of the Customer. In the event of any assignment, the Contractor remains secondarily liable for performance of the contract, unless the Customer expressly waives such secondary liability. The Customer may assign the Contract with prior written notice to Contractor of its intent to do so. 4.30 Antitrust Assignment. The Contractor and the State of Florida recognize that in actual economic practice, overcharges resulting from antitrust violations are in fact usually borne by the State of Florida. Therefore, the contractor hereby assigns to the State of Florida any and all claims for such overcharges as to goods, materials or services purchased in connection with the Contract. 4.31 Dispute Resolution. Any dispute concerning performance of the Contract shall be decided by the Customer's designated contract manager, who shall reduce the decision to writing and serve a copy on the Contractor. The decision shall be final and conclusive unless within twenty one (21) days from the date of receipt, the Contractor files with the Customer a petition for administrative hearing. The Customer’s decision on the petition shall be final, subject to the Contractor’s right to review pursuant to Chapter 120 of the Florida Statutes. Exhaustion of administrative remedies is an absolute condition precedent to the Contractor's ability to pursue any other form of dispute resolution; provided, however, that the parties may employ the alternative dispute resolution procedures outlined in Chapter 120. Without limiting the foregoing, the exclusive venue of any legal or equitable action that arises out of or relates to the Contract shall be the appropriate state court in Leon County, Florida; in any such action, Florida law shall apply and the parties waive any right to jury trial. 4.32 Employees, Subcontractors, and Agents. All Contractor employees, subcontractors, or agents performing work under the Contract shall be properly trained technicians who meet or exceed any specified training qualifications. Upon request, Contractor shall furnish a copy of technical certification or other proof of qualification. All employees, subcontractors, or agents performing work under the Contract must comply with all security and administrative requirements of the Customer and shall comply with all controlling laws and regulations relevant to the services they are providing under the Contract. The State may conduct, and the Contractor shall cooperate in, a security background check or otherwise assess any employee, subcontractor, or agent furnished by the Contractor. The State may refuse access to, or require replacement of, any personnel for cause, including, but not limited to, technical or training qualifications, quality of work, change in security status, or non-compliance with a Customer’s security or other requirements. Such approval shall not relieve the Contractor of its obligation to perform all work in compliance with the Contract. The State may reject and bar from any facility for cause any of the Contractor’s employees, subcontractors, or agents. 4.33 Security and Confidentiality. The Contractor shall comply fully with all security procedures of the United States, State of Florida and Customer in performance of the Contract. The Contractor shall not divulge to third parties any confidential information obtained by the Contractor or its agents, distributors, resellers, subcontractors, officers or employees in the course of performing Contract work, including, but not limited to, security procedures, business operations information, or commercial proprietary information in the possession of the State or Customer. The Contractor shall not be required to keep confidential information or material that is publicly available through no fault of the Contractor, material that the Contractor developed independently without relying on the State’s or Customer’s confidential information, or material RFP No. 10-475-000-J Medical and Dental Supplies Page 30 that is otherwise obtainable under State law as a public record. To insure confidentiality, the Contractor shall take appropriate steps as to its personnel, agents, and subcontractors. The warranties of this paragraph shall survive the Contract. 4.34 Contractor Employees, Subcontractors, and Other Agents. The Customer and the State shall take all actions necessary to ensure that Contractor's employees, subcontractors and other agents are not employees of the State of Florida. Such actions include, but are not limited to, ensuring that Contractor's employees, subcontractors, and other agents receive benefits and necessary insurance (health, workers' compensations, and unemployment) from an employer other than the State of Florida. 4.35 Insurance Requirements. During the Contract term, the Contractor at its sole expense shall provide commercial insurance of such a type and with such terms and limits as may be reasonably associated with the Contract. Providing and maintaining adequate insurance coverage is a material obligation of the Contractor. Upon request, the Contractor shall provide certificate of insurance. The limits of coverage under each policy maintained by the Contractor shall not be interpreted as limiting the Contractor’s liability and obligations under the Contract. All insurance policies shall be through insurers authorized or eligible to write policies in Florida. 4.36 Warranty of Authority. Each person signing the Contract warrants that he or she is duly authorized to do so and to bind the respective party to the Contract. 4.37 Warranty of Ability to Perform. The Contractor warrants that, to the best of its knowledge, there is no pending or threatened action, proceeding, or investigation, or any other legal or financial condition, that would in any way prohibit, restrain, or diminish the Contractor’s ability to satisfy its Contract obligations. The Contractor warrants that neither it nor any affiliate is currently on the convicted vendor list maintained pursuant to section 287.133 of the Florida Statutes, or on any similar list maintained by any other state or the federal government. The Contractor shall immediately notify the Customer in writing if its ability to perform is compromised in any manner during the term of the Contract. 4.38 Notices. All notices required under the Contract shall be delivered by certified mail, return receipt requested, by reputable air courier service, or by personal delivery to the agency designee identified in the original solicitation, or as otherwise identified by the Customer. Notices to the Contractor shall be delivered to the person who signs the Contract. Either designated recipient may notify the other, in writing, if someone else is designated to receive notice. 4.39 Leases and Installment Purchases. Prior approval of the Chief Financial Officer (as defined in Section 17.001, F.S.) is required for State agencies to enter into or to extend any lease or installment-purchase agreement in excess of the Category Two amount established by section 287.017 of the Florida Statutes. 4.40 Prison Rehabilitative Industries and Diversified Enterprises, Inc. (PRIDE). Section 946.515(2), F.S. requires the following statement to be included in the solicitation: "It is expressly understood and agreed that any articles which are the subject of, or required to carry out, the Contract shall be purchased from the corporation identified under Chapter 946 of the Florida Statutes (PRIDE) in the same manner and under the same procedures set forth in section 946.515(2) and (4) of the Florida Statutes; and for purposes of the Contract the person, company, or other business entity carrying out the provisions of the Contract shall be deemed to be substituted for the agency insofar as dealings with such corporation are concerned." RFP No. 10-475-000-J Medical and Dental Supplies Page 31 Additional information http://www.pridefl.com. about PRIDE and the products it offers is available at 4.41 Products Available from the Blind or Other Handicapped. Section 413.036(3), F.S. requires the following statement to be included in the solicitation: "It is expressly understood and agreed that any articles that are the subject of, or required to carry out, this contract shall be purchased from a nonprofit agency for the Blind or for the Severely Handicapped that is qualified pursuant to Chapter 413, Florida Statutes, in the same manner and under the same procedures set forth in section 413.036(1) and (2), Florida Statutes; and for purposes of this contract the person, company, or other business entity carrying out the provisions of this contract shall be deemed to be substituted for the State agency insofar as dealings with such qualified nonprofit agency are concerned." Additional information about the designated nonprofit agency and the products it offers is available at http://www.respectofflorida.org. 4.42 Modification of Terms. The Contract contains all the terms and conditions agreed upon by the parties, which terms and conditions shall govern all transactions between the Customer and the Contractor. The Contract may only be modified or amended upon mutual written agreement of the Customer and the Contractor. No oral agreements or representations shall be valid or binding upon the Customer or the Contractor. No alteration or modification of the Contract terms, including substitution of product, shall be valid or binding against the Customer. The Contractor may not unilaterally modify the terms of the Contract by affixing additional terms to product upon delivery (e.g., attachment or inclusion of standard preprinted forms, product literature, “shrink wrap” terms accompanying or affixed to a product, whether written or electronic) or by incorporating such terms onto the Contractor’s order or fiscal forms or other documents forwarded by the Contractor for payment. The Customer's acceptance of product or processing of documentation on forms furnished by the Contractor for approval or payment shall not constitute acceptance of the proposed modification to terms and conditions. 4.43 Cooperative Purchasing. Pursuant to their own governing laws, and subject to the agreement of the Contractor, other entities may be permitted to make purchases at the terms and conditions contained herein. Non-Customer purchases are independent of the agreement between Customer and Contractor, and Customer shall not be a party to any transaction between the Contractor and any other purchaser. State agencies wishing to make purchases from this agreement are required to follow the provisions of s. 287.042(16) (a), F.S. This statute requires the Department of Management Services to determine that the requestor's use of the contract is cost-effective and in the best interest of the State. 4.44 Waiver. The delay or failure by the Customer to exercise or enforce any of its rights under this Contract shall not constitute or be deemed a waiver of the Customer’s right thereafter to enforce those rights, nor shall any single or partial exercise of any such right preclude any other or further exercise thereof or the exercise of any other right. 4.45 Annual Appropriations. The State’s performance and obligation to pay under this contract are contingent upon an annual appropriation by the Legislature. 4.46 Execution in Counterparts. The Contract may be executed in counterparts, each of which shall be an original and all of which shall constitute but one and the same instrument. 4.47 Severability. RFP No. 10-475-000-J Medical and Dental Supplies Page 32 If a court deems any provision of the Contract void or unenforceable, that provision shall be enforced only to the extent that it is not in violation of law or is not otherwise unenforceable and all other provisions shall remain in full force and effect. The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 33 SECTION 5.0 SPECIAL CONTRACT CONDITIONS NOTE: SPECIAL CONTRACT CONDITIONS CONTAINED IN SECTION 5.0 WILL SUPERSEDE AND SUPPLEMENT GENERAL CONTRACT CONDITIONS (PUR 1000) CONTAINED IN SECTION 4.0. 5.1 Customer Service Support Contractor(s) shall have a single point of contact for customer support. Prompt notification of any change in the contact person is required. 5.2 Insurance Requirements During the Contract term, the Contractor at its sole expense shall provide commercial insurance of such a type and with such terms and limits as may be reasonably associated with the Contract, which, at a minimum, shall be: workers’ compensation and employer’s liability insurance per Florida statutory limits (currently $100,000 per accident, $100,000 per person, and $500,000 policy aggregate) covering all employees engaged in any Contract work; commercial general liability coverage on an occurrence basis in the minimum amount of $500,000 (defense cost shall be in excess of the limit of liability), naming the State as an additional insured; and automobile liability insurance covering all vehicles, owned or otherwise, used in the Contract work, with minimum combined limits of $500,000, including hired and non-owned liability, and $5,000 medical payment. Providing and maintaining adequate insurance coverage is a material obligation of the Contractor and is of the essence of the Contract. The Contract shall not limit the types of insurance Contractor may desire to obtain or be required to obtain by law. The limits of coverage under each policy maintained by the Contractor shall not be interpreted as limiting the Contractor’s liability and obligations under the Contract. All insurance policies shall be through insurers authorized to write policies in Florida. 5.3 Contract Revisions Any revisions to the originally approved contract information must be authorized in writing by State Purchasing prior to implementation. Failure to obtain State Purchasing approval prior to implementation may result in termination. 5.4 Compliance with Laws The Contractor shall comply with all laws, rules, codes, ordinances, and licensing requirements that are applicable to the conduct of its business, including those of federal, State, and local agencies having jurisdiction and authority. By way of non-exhaustive example, Chapter 287 of the Florida Statutes and Chapter 60A-1of the Florida Administrative Code govern the Contract. By way of further nonexhaustive example, the Contractor shall comply with section 247A(e) of the Immigration and Nationalization Act, the Americans with Disabilities Act, and all prohibitions against discrimination on the basis of race, religion, sex, creed, national origin, handicap, marital status, or veteran’s status. Violation of such laws shall be grounds for Contract termination. 5.5 Price Adjustments RFP No. 10-475-000-J Medical and Dental Supplies Page 34 No price escalations (increases) will be permitted during the three (3) year term of this contract. Price de-escalation (decrease) is permissible at any time during the contract term. The Department reserves the right to require a decrease based on industry pricing indicators (PPI and CPI) Contractors may request a price increase at renewal, if any (see Section 4.26). Price increase requests must be based on the Producer Price Index (PPI) Table Containing PPI-U All Items Indexes and Annual Percent Changes, http://data.bls.gov/PDQ/servlet/SurveyOutputServlet;jsessionid=f030761aed77$3FB$0A$ and may not exceed 4 percent. The price increase request must be submitted 60 days before the expiration of the Contract, to the Contract Administrator, in writing and substantiated by a copy the appropriate (meaning specific to that Contractor’s commodity code) PPI index reports. http://www.bls.gov/news.release/ppi.toc.htm 5.6 Punch out Catalogues and e-Invoicing Awarded contractors are not encouraged to create punch out catalogues in MFMP for this contract. Awarded contractors are encouraged to participate in e-Invoicing. Please contact the MFMP Customer Service Desk at 1-866-352-3776, for assistance. 5.7 Emergency Contact The Contractor will provide the Customer’s Contract Administrator with a 24/7 Emergency Contact. 5.8 Performance and Payment Bonds The authority and responsibility for requesting performance and payment bonds rests with the Customer. Under this contract, the Customer may request a performance and payment bond as deemed necessary by the size of the job. 5.9 Contractor Performance Each Contractor's performance will be evaluated on an ongoing basis through the following procedures: 1. Customers will have access to a Contract Survey (Performance Evaluation) through the State Term Contract web site to rate their satisfaction with the overall contract and the contractor, 2. Executive agency customers will have access to the Vendor Performance Tracking System through MyFloridaMarketPlace to rate their satisfaction following every completed order. 3. Contractors will be evaluated on the number and severity of complaints received from contract users. (Complaint to Vendor Form PUR 7017.) 4. Timely submission of Monthly Transaction Reports and applicable fees will be a key factor in this evaluation. See Section 4.14, Transaction Fee. Reports and Fees are to be submitted by the 15th of each calendar month. 5. Volume of sales will be reviewed as part of Contractor performance. RFP No. 10-475-000-J Medical and Dental Supplies Page 35 5.10 Delays and Complaints Service delays and service complaints will be monitored on a continual basis. Documented inability to perform under the conditions of the contract may result in default proceedings and/or termination. 5.11 Sales Requirements Sales will be reviewed on a quarterly basis. Should no sales be recorded in two consecutive contract quarters, the contract supplier may be selected for termination. State Purchasing reserves the right to require awarded Respondents to submit detailed sales reports upon request. See General Contract Conditions, PUR 1000, Transaction Fee, Section 4.14 for MONTHLY reporting requirements. The intent of the sales requirement is to maintain the value of the contract. Any termination resulting solely from this requirement would be for convenience and would not impact the contractor’s ability to conduct future business with the state. 5.12 Contract Sales Summary Report The following data must be reported to the Department on a quarterly contract basis (See blank form in Section 7: Quarterly State Term Contract Sales Summary Report Attachment 8.) The Report shall include: Contractor’s Name Reporting Period Total dollar value of purchases per quarter separated by individual State Agency and Eligible User totals. Failure to provide quarterly sales reports, including a “no sales” report if no sales were made, within thirty (30) calendar days following the end of each contract quarter, may result in the contract supplier being found in default and cancellation of the contract by the Department. Initiation and submission of the Contract Sales Summaries are to be the responsibility of the Contractor without prompting or notification by the Contract Administrator. The Contractor will submit the completed Contract Sales Summary forms by email to the Contract Administrator. 5.13 State Objectives Within thirty (30) calendar days following award of the Contract, if awarded, the successful Respondent shall submit plans addressing each of the State’s five (5) objectives listed below, to the extent applicable to the items / services covered by this solicitation. 5.13.1 Diversity The State of Florida is committed to supporting its diverse business industry and population through ensuring participation by minority- and women-owned business enterprises in the economic life of the state. The State of Florida Mentor Protégé Program connects minority- and women-owned businesses with private corporations for business development mentoring. We RFP No. 10-475-000-J Medical and Dental Supplies Page 36 strongly encourage firms doing business with the State of Florida to consider this initiative. For more information on the Mentor Protégé Program, please contact the Office of Supplier Diversity at (850) 487-0915. It is vital that small, minority, women-owned and veteran-owned business enterprises participate in the State’s procurement process as both prime Contractors and Subcontractors under prime Contracts. Small, minority, and women-owned businesses are strongly encouraged to submit replies to this solicitation. The Contractor shall submit documentation addressing Diversity and describing the efforts being made to encourage the participation of small, minority, women-owned and veteran-owned businesses. Information on Certified Minority Business Enterprises (CMBE) is available from the Office of Supplier Diversity at: http://dms.myflorida.com/other_programs/office_of_supplier_diversity_osd/. 5.13.2 Environmental Considerations The State supports and encourages initiatives to protect and preserve our environment. The Contractor shall submit as part of any response the Contractor’s plan to support the procurement of products and materials with recycled content, and the intent of Section 287.045, Florida Statutes. The Contractor shall also provide a plan for reducing and or handling of any hazardous waste generated by Contractor’s company. Reference Rule 62-730.160, Florida Administrative Code. It is a requirement of the Florida Department of Environmental Protection that a generator of hazardous waste materials that exceeds a certain threshold must have a valid and current Hazardous Waste Generator Identification Number. This identification number shall be submitted as part of Contractor’s explanation of its company’s hazardous waste plan and shall explain in detail its handling and disposal of this waste. 5.13.3 Certification of Drug-Free Workplace Program The State supports and encourages initiatives to keep the workplaces of Florida’s Suppliers and Contractors drug free. Section 287.087 of the Florida Statutes provides that, where identical tie responses are received, preference shall be given to a response received from a Respondent that certifies it has implemented a drug-free workforce program. If applicable, Respondent shall certify that the Respondent has a drug-free workplace program using the Certification of DrugFree Workplace form included in Section 7.5 of the solicitation. The Contractor shall describe how it will address the implementation of a drug free workplace in offering the items of the solicitation. 5.13.4 Products Available from the Blind or Other Handicapped (RESPECT) The State supports and encourages the gainful employment of citizens with disabilities. It is expressly understood and agreed that any articles that are the subject of, or required to carry out, this Contract shall be purchased from a nonprofit agency for the blind or for the severely handicapped that is qualified pursuant to Chapter 413, Florida Statutes, in the same manner and under the same procedures set forth in Section 413.036(1) and (2), Florida Statutes; and for purposes of this Contract the person, firm, or other business entity carrying out the provisions of this Contract shall be deemed to be substituted for the state agency insofar as dealings with such qualified nonprofit agency are concerned. Additional information about the designated nonprofit agency and the products it offers is available at http://www.respectofflorida.org. The Contractor shall describe how it will address the use of RESPECT in offering the items of the solicitation. RFP No. 10-475-000-J Medical and Dental Supplies Page 37 5.13.5 Prison Rehabilitative Industries and Diversified Enterprises, Inc. (PRIDE) The State supports and encourages the use of Florida correctional work programs. It is expressly understood and agreed that any articles which are the subject of, or required to carry out, this Contract shall be purchased from the corporation identified under Chapter 946, F.S., in the same manner and under the same procedures set forth in Section 946.515(2), and (4), F.S.; and for purposes of this contract the person, firm, or other business entity carrying out the provisions of this Contract shall be deemed to be substituted for this agency insofar as dealings with such corporation are concerned. Additional information about PRIDE and the products it offers is available at http://www.pride-enterprises.org/. The Contractor shall describe how it will address the use of PRIDE in offering the items of the solicitation. The balance of this sheet is blank intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 38 SECTION 6.0 TECHNICAL SPECIFICATIONS 6.1 Purpose The Department of Management Services, Division of State Purchasing, invites interested companies to submit proposals in accordance with the solicitation documents, to provide state agencies and other eligible users with competent, experienced and professional Medical and Dental Supply manufacturers and/or distributers. The Department’s goal is to provide contract users with the best value in medical and dental supplies. 6.2 Experience, Capabilities and Certifications Respondents are required to provide five (5) references on the Reference Form (Attachment 2) with a minimum of two (2) being either large accounts (over $100,000) or contracts with a governmental entity. Use Attachment 4 to illustrate your company’s reach and capabilities. 6.3 Scope The purpose of this Request for Proposal (RFP) is to establish a three (3) year, state term contract for Medical and Dental Supplies, with the potential for renewal in accordance with F.S. 287.057(1)(a) and if the Department determines renewal to be in the best interest of the State. Each Customer may have more than one location and in different regions of the state. Respondents are asked to submit proposals in accordance with this diversity and your corporate capabilities. For the purpose of this RFP, a “Core Item” is any item on the lists provided in Section 7. Respondents are to enter the list price, standard price, the Manufacturer’s Suggested Retail Price (MSRP) and or catalogue price and provide a discounted percentage and price for each Core Item they are capable of providing. Should a core item become discontinued by the manufacturer, it will be removed from the “Core Item” list. Its replacement, if any, will become a “Non Core Item.” Contractors are to notify the Department in writing when an item is being discontinued. The notification shall include validation from the Manufacturer. “Non Core” items are anything else your company may sell. “Non Core” items include but are not limited to other tools, equipment, “one time buys”, other supplies, new products, and replacements for discontinued items. All products under this contract shall be new, unused and available. All products shall be manufactured in accordance with the United States Consumer Products Safety Commission http://www.cpsc.gov/ http://www.cpsc.gov/. . All medical electrical products are to comply with, at minimum with UL 60601-1 http://ulstandardsinfonet.ul.com/scopes/scopes.asp?fn=606011.html No products, either on the Core or the Non Core items lists are to be refurbished (cleaned up, re-sharpened, re-painted, re-cased, re-calibrated, etc.) or remanufactured (the disassembly of previously sold, non working units, salvaging working parts and manufacturing a separate unit using the salvaged parts) unless specifically requested by the Customer, in writing. RFP No. 10-475-000-J Medical and Dental Supplies Page 39 Respondents are also asked to provide a discount percentage off list price, standard price, MSRP and or catalogue price for the “Non Core” items by category. These stated discounts are to be the minimum discount offered on the item; Customers are encouraged to request larger discounts from any awarded Respondent. Offering a discount percentage on “Non Core” items is not required as part of a Respondents bid. Awarded Respondents (Contractors) not offering a discount on Non Core items will not be permitted to sell Non Core items under this contract. Respondents may offer substitute products which are equal to or better than those products listed on the Excel® spreadsheet. Those Respondents offering substitutes must provide the specification sheets on both the requested product and the substitute, in their response. When offering a substitute, enter a Y (yes) or a check mark in the Substitute column and change the description. Make sure the product count per box and per case is included with the description. Awarded Respondents, if any, are to advise Customers in advance of any applicable HAZMAT shipping requirements and associated costs. The Customer has the option to negotiate the associated costs or remove the item from the order. Current pricing and Non Core discounts on the existing contract can be reviewed at; http://dms.myflorida.com/business_operations/state_purchasing/vendor_information/state_contr acts_agreements_and_price_lists/state_term_contracts/medical_and_dental_supply The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 40 SECTION 7.0 FORMS AND ATTACHMENTS FORMS AND ATTACHMENTS ARE ACCESSIBLE IN THE MYFLORIDAMARKETPLACE SOURCING TOOL. Attachment 1: Proposal Checklist Form Attachment 2: Reference Summaries Attachment 3: Experience Certification Form Attachment 4: Respondents Capability Form with District Map Attachment 5: Ordering Instructions Form Attachment 6: Certification of Drug Free Workplace Program Attachment 7: Excel® Price Sheets – a copy included herein and as a separate, interactive document in MFMP Attachment 8: Quarterly Contract Sales Summary Report Contract Form It is NOT necessary for you to fill all lines on the Price Sheet – enter only the items you wish to bid on. Do not delete items/lines you are not bidding on – leave the spreadsheet intact. Altered spreadsheets cannot be scored. The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 41 Attachment 1 Proposal Checklist Form This Proposal Preparation Checklist is provided to assist potential Contractors with assembling their proposal but does not relieve the potential Contractors of responsibility for ensuring that all requirements of the solicitation are included with the submittal. Attachment 1 (Proposal Checklist Form) does not need to be included with your response. All numbered attachments are available as separate downloadable forms in the MyFloridaMarketPlace Sourcing Tool. Respondents are to download the attachments from Sourcing, or VBS, complete them and save unto a CD for submittal. Responses as required in Section 3.8 Submittal Content Attachment 2 Reference Summaries Attachment 3 Experience Certification Form Attachment 4 Respondents Capability Form Attachment 5 Ordering Instructions Attachment 6 Certification of Drug Free Workplace Program Attachment 7 Excel® Price Sheet, included herein and as a separate document Names and contact information of key management and contract personnel A copy of Liability Insurance declaration page A copy of your company’s financial reports or Dun and Bradstreet report (or equivalent), no older than 6 months A copy of the signed contract with seal, if applicable. Make sure that your 3 DCs are readable by any computer using Windows ® Make sure that the Ordering Instructions sheet is typed – not hand printed. Save the Excel Price Sheets as Excel 97-2003 Workbooks and as a separate file on your CDs. Altered spreadsheets cannot be scored. The Department encourages Respondents to ensure compliance with MyFloridaMarketPlace Transaction Fee reporting and payment requirements prior to your submittal. Respondents that are not compliant with transaction fee reporting and payment requirements may be determined to be non-responsible. RFP No. 10-475-000-J Medical and Dental Supplies Page 42 Attachment 2 Reference Summaries . Provide a minimum of five references; large governmental and/or commercial accounts are preferred. No actual form is provided. Information shall include: Customer name, customer contact name, address, phone number, fax number and email address. Description of services provided. Specific dates of service. Average cost per job/task, or total cost of project. RFP No. 10-475-000-J Medical and Dental Supplies Page 43 Attachment 3 Experience Certification Form Attention: Department of Management Services From: (insert Respondent’s name) RE: RFP 10-475-000-J Medical and Dental Supplies This form will serve to confirm that _____________________________ (insert Company Name) has a minimum of five (5) years experience in manufacturing and/or distributing quality medical and dental supplies. Signed: ________________________________________ Title: ________________________________________ Company: ______________________________________ Sworn to a subscribed before me this _____ day of _____________, 20___ Notary Public: ___________________________________ My Commission Expires: ___________________________ The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 44 Attachment 4 Respondents Capability Form Please use this form as a guideline for your narrative response. 1. Can your company provide quality medical and dental supplies statewide? 2. Does your company offer free or low cost local deliveries? Please explain. 3. Does your company offer free inside delivery? 4. Please detail and identify the purchasing district(s) or individual counties your company is capable of servicing. (See district map on the next page) 5. Provide your company’s State of Florida license number. 6. Does your company have the capability to respond quickly to a disaster/emergency? Please explain. 7. Explain your company’s VMI (Vendor Managed Inventory) capabilities. 8. Provide a copy of your company’s liability policy – declaration page only - if your company does not have the necessary insurance coverage at the time of response submittal, please note this in the response and refer to section 5.2. The balance of this sheet is blank, intentionally RFP No. 10-475-000-J Medical and Dental Supplies Page 45 RFP No. 10-475-000-J Medical and Dental Supplies Page 46 Attachment 5 Ordering Instructions Form PLEASE TYPE Contractor name: ______ ____________________________________________ FEID NUMBER: Please identify the person who will be responsible for administering the Contract on your behalf if award is made, and include an emergency contact phone number: Name: Title: Street Address: E-mail Address: Phone Number(s): Fax Number: If the person responsible for answering questions about the solicitation is different from the person identified above, please provide the same information for that person. Name: Title: Street Address: E-mail Address: Phone Number(s): Fax Number: Ordering Information: Please provide the following information about where Customers should direct orders. You must provide a regular mailing address. If equipped to receive purchase orders electronically, you may also provide an Internet address. Spurs Information Number: Name: Title: Street Address or P.O. Box: City, State, Zip: Phone Number: Toll Free Number: Ordering Fax Number: Internet Address: Federal ID Number: Remit Address: City, State, Zip: NOTE: Duplicate as necessary for multiple ordering locations. RFP No. 10-475-000-J Medical and Dental Supplies Page 47 Attachment 6 Certification of Drug Free Workplace Program Section 287.087 of the Florida Statutes provides that, where identical tie bids are received, preference shall be given to a bid received from a bidder that certifies it has implemented a drug-free workforce program. Please sign below and return this form to certify that your business has a drug-free workplace program. 1) Publish a statement notifying employees that the unlawful manufacture, distribution, dispensing, possession or use of a controlled substance is prohibited in the workplace and specifying the actions that will be taken against employees for violations of such prohibition. 2) Inform employees about the dangers of drug abuse in the workplace, the business's policy of maintaining a drug-free workplace, any available drug counseling, rehabilitation and employee assistance programs and the penalties that may be imposed upon employees for drug abuse violations. 3) Give each employee engaged in providing the commodities or contractual services that are under Bid a copy of the statement specified in subsection (1). 4) In the statement specified in subsection (1), notify the employees, as a condition of working on the commodities or contractual services that are under Bid, the employee will abide by the terms of the statement and will notify the employer of any conviction of, or plea of guilty or nolo contendere to, any violation of Chapter 893 or of any controlled substance law of the United States or any State, for a violation occurring in the workplace no later than five (5) days after such conviction. 5) Impose a sanction on, or require the satisfactory participation in a drug abuse assistance or rehabilitation program if such is available in the employee's community by any employee who is so convicted. 6) Make a good faith effort to continue to maintain a drug-free workplace through implementation of this section. As the person authorized to sign the statement, I certify that this company complies fully with the above requirements. False statements are punishable by law. RESPONDENT’S NAME: By: Authorized Signature Print Name and Title (04/02) RFP No. 10-475-000-J Medical and Dental Supplies Page 48 Attachments 7 Price Sheet Attachment 7 is also separate Excel® spreadsheets, pre loaded with the pricing formula, that can be downloaded from the MyFloridaMarketPlace Sourcing Tool. The Price sheet contained herein is for reference only. See Section 3.6 Submittal of Response. Please review booth the Medical and the Dental spreadsheets as there may be some overlap. Medical Supplies Manufacturer 3M Mfg SKU 1583 Description 1860 2 3M 1222-1 3 3M 1530-1 4 List price Discount off List Price to State $0.00 0% 0.00 $0.00 0% 0.00 Bandage, Elastic, Coban, 3' X 5Yd/RL, 3M, 1583, 24RL/CS 1 3M UoM Distributer SKU Mask, Respirator, Particular, Type N95, Regular, 3M, 1860, 20EA/BX, 6BX/CS Tape, Indicator, Beige, Sterilization, 1" X 60Yd, 3M, 1222-1, 18RL/CS 0.00 Tape, Surgical, Paper, Hypoallergenic, 1" X 10Yd, 3M, 1530-1, 12EA/BX, 10BX/CS 0.00 1538-1 Tape, Surgical, Silk-like, Hypoallergenic, 1" X 10Yd, 3M, 1538-1, 12EA/BX, 10BX/CS 4509-1629 6 Dressing, Transparent, Waterproof, Orange Frame Style, 8" X 12", 3M, 4509-1629, 10EA/BX, 8BX/CS 0.00 5122-BX/100 7 Thermometer, Oral, Sterile, Fahrenheit, Sterile, Single-Use, Individually Wrapped, 3M, 5122BX/100, 100EA/BX, 20BX/CS 0.00 3M 5 3M 3M 3M 1624W 8 3M 1527-1 0.00 TEGADERM 23/8 X 23/4 1624W, 3M, 1624W,100EA/BX,4BX/CS 0.00 Tape, Transpore, 1", 3M Corp, 1527-1, 12/BX 9 0.00 3M 1626 Dressing, Tegaderm, 4", 3M, 1626, 50/Bx 10 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 49 Abbott 4565-01 Infusion Set, 23G, Butterfly Shape, Abbott, 4565-01, 40EA/BG, 3BG/CS 7983-02 Sodium Chloride, 0.9%, Injection, USP, Lifecare, 250 Ml, Abbott, 7983-02, 250ML/EA, 24EA/CS 11 Abbott 12 ABBOTT 4492-01 0.00 0.00 Infusion Set, Butterfly, Abbott, 4492-01, Ea 13 0.00 Allegiance 9545 Pack, Gown, Surgical, XL, Allegiance, 9545, 20EA/CS 14 0.00 Allegiance 0185-9103 15 ALLEGIANCE 3T6733B 16 ALLEGIANCE KBK90 Pack, Basic General Surgery, Disposable, Allegiance, 01859103, 8EA/CS 0.00 URETHRAL CATH TRAY RR/PVP, ALLEGIANCE, 3T6733B,1TRAY=1EA,20EA/CS 0.00 Catheter. Plastic, Urethral, Bard, Kbk90, Ea 17 0.00 ALLIED HEALTHCARE 64041 Mask, O2, Med Adult, Allied, 64041, Ea 18 0.00 ALLIED HEALTHCARE 33239 Nasal Cannual Adult Clear, Allied Healthcare, 33239, Ea 19 0.00 Allied Healthcare Products Inc 33604 20 Allied Healthcare Products Inc 61399 21 Allied Healthcare Products Inc 64200 22 ALMORE INTERNATIONAL INC AMBU INC. 2802 Shield, Full Face, w/ Velcro, Band, Anti-Fog Coating, Alpha Pro Tech, Inc. Rp., 2802, 25EA/BX, 4BX/CS 248 201 102 25 AMERICAN BIO MEDICAL CORP 5P1 26 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Tubing, Oxygen, 7', Allied Healthcare Products Inc, 64200, 50EA/CS Binocular Loupe With Lenses +5:00, Almore International Inc, 87000,Ea 24 0.00 Nebulizer, Complete, 6" Flex Tubing, Allied Healthcare Products Inc, 61399, 50EA/CS 87000 23 Alpha Pro Tech, Inc. Rp. Cannula, Nasal Pediatric, Clear w/ 7' Tubing, Allied Healthcare Products Inc, 33604, 50EA/CS 0.00 0.00 0.00 Ambu Rescue Key With Nylon Case, Ambu Inc., 248 201 102, Ea 0.00 Drug Screen, Rapid Test Kit, American Bio Medical Gr, 5P1,Ea 0.00 Page 50 American Diagnostic Corp. 922 27 American Diagnostic Corp. 0241-660N 28 American Diagnostic Corp. 720MM 29 American Diagnostic Corp. 720TDB 30 Sphygmomanometer, Latex, Adult, Black, American Diagnostic Corp., 922, EA 0.00 Stethoscope, Single Head, Navy, American Diagnostic Corp., 0241-660N, EA 0.00 Sphygmomanometer, Aneroid, Latex, Adult, Black, American Diagnostic Corp., 720MM, EA 0.00 Sphygmomanometer, Aneroid, Thigh, Latex, Black, American Diagnostic Corp., 720TDB, EA 0.00 732DB System, Multicuff, Four Cuffs, Latex, Navy, American Diagnostic Corp., 732DB, EA 752MLF 32 Sphygmomanometer, Aneroid, Mobile, Adult, Black, Latex Free, American Diagnostic Corp., 752MLF, EA 0.00 775-660MM 33 Kit, Sphygmomanometer, Aneroid, w/ Stethoscope, Latex, Navy, American Diagnostic Corp., 775-660MM, EA 0.00 41405-10757 34 CARTOON BAND-AID, AMERICAN WHITE CROSS, 41405-10757,100EA/BX, 12BX/CS 0.00 American Diagnostic Corp. 31 American Diagnostic Corp. American Diagnostic Corp. AMERICAN WHITE CROSS AMERICAN WHITE CROSS 1075033 35 AMERICAN WHITE CROSS 1307033 36 AMES 2161 0.00 Band aid, 3/4", Adhesive, American White Cross, 1075033, 100/Bx 0.00 Band aid, Adhesive Spot, 7/8", American White Cross, 1307033, 100/Bx 0.00 Multistix 10Sg Urine, Ames, 2161, Ea 37 0.00 AS026 38 Applicator Sticks, Regular, NonSterile, 6", Amsino International, Inc., AS026, 1000EA/BX, 20BX/CS 0.00 5300TN-024 39 Bandage, Co-Flex, Latex-Free, 3" X 5Yd, Andover Coated Products, 5300TN-024, 1EA/BX, 24BX/CS 0.00 Amsino International, Inc. Andover Coated Products ANSELL PERRY 17003 Gloves, Pf Sterile, Sz 7, Ansell Perry, 17003, 50/Bx 40 0.00 APEX-CAREX HEALTHCARE Crutch Tips, Large, Apex-Carex Healthcare, A715-Oo, Pr 41 0.00 ASO CORPORATION 5261-012 42 RFP No. 10-475-000-J Medical and Dental Supplies BANDAID CRAYON ADHES, ASO CORPORATION, 5261012,100EA/BX, 12BX/CS 0.00 Page 51 ASO CORPORATION 5293-012 43 ASO CORPORATION 5375-012 BANDAID NEON SPOT 7/, ASO CORPORATION, 5375012,100EA/BX, 12BX/CS MOO6418 MOORE BUTTERFLY CLOSURE MED, ASO CORPORATION, MOO6418,100EA/BX, 12BX/CS 44 ASO CORPORATION 45 ASO CORPORATION 3019-012 46 ASO CORPORATION 1019-012 47 ASO CORPORATION 4303-012 48 ASO CORPORATION 4302-012 49 ASO CORPORATION BANDAID GARFIELD 3/4, ASO CORPORATION, 5293012,100EA/BX, 12BX/CS M004018 50 0.00 0.00 0.00 BANDAGE 1X3 CLEAR ST, ASO CORPORATION, 3019012,100EA/BX, 12BX/CS 0.00 BANDAGE STRIP 1X3 PL, ASO CORPORATION, 1019012,100EA/BX, 12BX/CS 0.00 BANDG FLEXILET L/F 1, ASO CORPORATION, 4303012,100EA/BX, 12BX/CS 0.00 BAND FLEXILET L/F 3/, ASO CORPORATION, 4302012,100EA/BX, 12BX/CS 0.00 MOORE FABRIC STRIPS 3/4X3 LF, ASO CORPORATION, M004018,100EA/BX, 12BX/CS 0.00 M004019 MOORE FABRIC STRIPS 1X3 LF, ASO CORPORATION, M004019,100EA/BX, 12BX/CS M002022 52 MOORE SHEER PLAST 7/8 SPOTS LF, ASO CORPORATION, M002022,100EA/BX, 12BX/CS 0.00 M002018 53 MOORE SHEER PLASTIC 3/4X3 LF, ASO CORPORATION, M002018,100EA/BX, 12BX/CS 0.00 ASO CORPORATION 51 ASO CORPORATION ASO CORPORATION ASO CORPORATION M002019 54 ASO CORPORATION 2019-012 0.00 MOORE SHEER PLASTIC 1X3 LF, ASO CORPORATION, M002019,100EA/BX, 12BX/CS 0.00 Bandage, 1 X 3 Sheer, Aso Corporation, 2019-012, 100/Bx 55 0.00 ASO CORPORATION 4018-012 Bandage, 3/4 X 3, Fabric, Aso Corporation, 4018-012, 100/Bx 56 0.00 ASO CORPORATION 2022-012 Bandage, 7/8" Spot Sheer, Aso Corporation, 2022-012, 100/Bx 57 0.00 ASO CORPORATION 2016-012 Bandage, Xlg Strip, Aso Corporation, 2016-012, 50/Bx 58 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 52 ASO CORPORATION M002027 59 ASO CORPORATION 2021 Bandage, Sheer Plastic Mini Lf, Aso Corporation, M002027, 100/Bx 0.00 Band aids, Sheer Strip, 1X3, Aso, 2021, Cs 60 0.00 Associated Hygienic Prods*Inc MSCSE97000 61 Associated Hygienic Prods*Inc MSCSE97500 62 Avcor Healthcare Prod Inc 23602-041 63 B&F 64095 Brief, Ultra Absorbent, Core, Medium, 32" - 44", White, Associated Hygienic Prods*Inc, MSCSE97000, 12EA/BG, 8BG/CS Brief, Ultra Absorbent, Core, Large, 44" - 58", Blue, Associated Hygienic Prods*Inc, MSCSE97500, 12EA/BG, 6BG/CS Bandage, Compression, 4" X 186", Extra Economy, Avcor Healthcare Prod Inc, 23602-041, 72EA/CS 0.00 0.00 0.00 Mask, Pediatric, w/ Nebulizer & Tubing, B & F, 64095, 50EA/CS 64 0.00 B.BRAUN R520101 65 B.Braun - Mcgaw, Inc. 7190-L8000 66 B.Braun - Mcgaw, Inc. 7190-S80045264 67 B.Braun - Mcgaw, Inc. 7190-S80045384 68 B.Braun - Mcgaw, Inc. V1402 69 BANTA HEALTHCARE 9810856 SODIUM CHLOR .9% IRR 2F7124, B.BRAUN, R520101,16EA/CS 0.00 Solution, Injectable, 9% Sodium Chloride, Exclusive Container, 1000ML, B.Braun - Mcgaw, Inc., 7190-L8000, 12EA/CS Solution, Injectable, 9% Sodium Chloride, Pab Container, 100/150ML, B.Braun - Mcgaw, Inc., 7190-S8004-5264, 64EA/CS Solution, Injectable, 9% Sodium Chloride, Pab Container, 50/100ML, B.Braun - Mcgaw, Inc., 7190-S8004-5384, 84EA/CS Set, Administering, Control Clamp, 2 Piece Male Luer Lock, 14ML, B.Braun - Mcgaw, Inc., V1402, 50EA/CS 0.00 0.00 0.00 0.00 Capes, 3 Ply White, Banta Healthcare, 9810856, 100/Cs 70 0.00 BANTA HEALTHCARE 96-9153 Cotton Balls, Medium, Banta Healthcare, 96-9153, 2000/Ct 71 0.00 BANTA HEALTHCARE 96-9152 Cotton Balls, Large, Banta Healthcare, 96-9152, 1000/Ct 72 0.00 BANTA HEALTHCARE 9810824 73 RFP No. 10-475-000-J Medical and Dental Supplies Drape Sheet 40X48 White, Banta Healthcare, 9810824, 100/Ea 0.00 Page 53 BANTA HEALTHCARE 9810826 74 BANTA HEALTHCARE 919355 Drape Sheet, 40X60, White, Banta Healthcare, 9810826, 100/Ea 0.00 Pillow Case, White Fabric, Banta, 919355, Bx 75 0.00 BANTA HEALTHCARE 981002 Table Paper, 18" Crepe, Banta, 981002, 12/Cs 76 0.00 BANTA HEALTHCARE 9810891 Table Paper, 18" Smooth, Banta, 9810891, 12/Cs 77 0.00 BANTA HEALTHCARE 981004 Table Paper, 21" Crepe, Banta, 981004, Banta, 12/Cs 78 0.00 BANTA HEALTHCARE 916143 79 BANTA HEALTHCARE 20634 Table Paper, Prem Crepe, Banta, 916143, 916143, 12/Cs/Bx 0.00 Thermometer Sheath, Banta, 20634, Bx 80 0.00 BANTA HEALTHCARE 9810865 Towel, Professional, 13X18, 2Ply, Banta, 9810865, 500/Cs 81 0.00 Banta Healthcare Products, Inc 9223 82 Banta Healthcare Products, Inc 908272 83 Banta Healthcare Products, Inc 911418 84 Banta Healthcare Products, Inc 9810892 85 BARD 0615-898314 Cup, Paper, 4OZ, Waxed, Banta Healthcare Products, Inc, 9223, 1000EA/CS 0.00 Gauze Sponge, Sterile, 2S, 4" X 4", 12Ply, High Quality, Banta Healthcare Products, Inc, 908272, 2EA/PK, 25PK/BX, 24BX/CS Table Paper, Exam, Crepe, 18" X 125', Tidisaurus, Banta Healthcare Products, Inc, 911418, 1EA/BX, 6BX/CS 0.00 0.00 Table Paper, Smooth, 21" X 200', Banta Healthcare Products, Inc, 9810892, 12RL/CS 0.00 Foley Cath Tray, 14Fr With U.D. Bag, Bard, 0615-898314, Bx 86 0.00 Baxter Healthcare 2C5417 IV Solution Set, Basic, One Injection Site, Male Luer Slip Adapter, 10 Drops/ML, 68" (1.7m), Baxter Healthcare, 2C5417, 48EA/CS 2C6537 IV Tubing Set, 110", 10 Drops/ML, Baxter Healthcare, 2C6537, 48EA/CS 87 Baxter Healthcare 88 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 Page 54 Baxter Healthcare 2M8063baxter 6201 89 BAXTER HEALTHCARE 2F7123 90 Baxter Healthcare 2N3399 IV Pump, Flow Guard, Yellow, Baxter Healthcare, 2M8063baxter6201, EA 0.00 SODIUM CHLR 0.9% IRR 2F7123P, BAXTER HEALTHCARE, 2F7123,1/EA 0.00 Luer Lock Injection Site, Baxter, 2N3399 91 0.00 Mask, Adult Oxygen 7", Baxter Healthcare, 1203, Ea BAXTER HEALTHCARE 92 0.00 Bayer 2163 93 Bayer 3942 Test Strip, Reagent, Urinalysis, Multistix 9SG, Bayer, 2163, 100EA/BTL, 1BTL/BX, 24BX/CS 0.00 Test Strip, Glucose, Elite, Bayer, 3942, 100EA/BX, 16BX/CS 94 0.00 Bayer 6516F Analyzer, Chemistry, Urine, Clinitek, Bayer, 6516F, EA 95 0.00 BCI International*** 3044 96 BCI International*** 3401-000 97 Beaumont Products Inc 633712928 98 Beaumont Products Inc 632712303/7760 99 Beaumont Products Inc 632712942/7756 100 Beckman Coulter Inc 62115 101 BECKMAN COULTER INC 43025 102 Beckman Coulter Inc 60151 103 Becton Dickinson 4893 104 RFP No. 10-475-000-J Medical and Dental Supplies Oximetry, Finger Sensor, Standard, BCI International, 3044, EA 0.00 Pulse Oximeter, Hand-Held, Finger Print, BCI International, 3401-000, EA 0.00 Cleaner, Germicidal, Deodorizer, One Gallon, Beaumont Products Inc, 633712928, 1GL/EA, 4EA/CS 0.00 Lotion, Hand Sanitizing, Instant Citrus, 8oz, Beaumont Products Inc, 632712303/7760, 12EA/CS 0.00 Lotion, Hand Sanitizing, Instant Citrus, 4oz, Beaumont Products Inc, 632712942/7756, 24EA/CS 0.00 Developer, Hemoccult, Solution, 15ML, Sterile, Beckman Coulter Inc, 62115, 20EA/CS 0.00 ICON 25HCG PREG TEST 43025, BECKMAN COULTER INC, 43025,4BX/CS 0.00 Hemoccult Single Slide Test, Beckman Coulter, 60151, 100/BX 0.00 Needle Holder, Standard, Becton Dickinson, 4893, 72EA/BX, 10BX/CS 0.00 Page 55 Becton Dickinson 207378 105 Becton Dickinson 300467 106 Becton Dickinson 300470 107 Becton Dickinson Becton Dickinson 305460 Collector, Sharps, Clear Top/Red, 8QT, Regular Funnel, Nest able, Becton Dickinson, 305460, 24EA/CS 305471 Collector, Sharps, Red, 3.2QT, Vertical Entry, Becton Dickinson, 305471, 36EA/CS 305551 Collector, Sharps, Clear, 5.4QT, Horizontal Drop, Next Generation, Patient Room, Becton Dickinson, 305551, 20EA/CS 110 Becton Dickinson 111 Becton Dickinson 309604 112 Becton Dickinson 328230 113 Becton Dickinson 367283 114 Becton Dickinson 367285 115 Becton Dickinson 371610 116 Becton Dickinson 371615 117 Becton Dickinson 383323 118 Becton Dickinson 387226 119 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Collector, Sharps, Red, 8.2QT, Large, Non-Vented, Becton Dickinson, 300470, 12EA/CS Collector, Sharps, Pearl, 5.4QT, Side Entry, Nest able, Becton Dickinson, 305444, 20EA/CS 109 0.00 Collector, Sharps, Red, 6.9QT, Medium, Non-Vented, Becton Dickinson, 300467, 12EA/CS 305444 108 Becton Dickinson Bandage, Elastic, Compression, 6", Stretches to 180", Becton Dickinson, 207378, 10EA/BX, 6BX/CS 0.00 0.00 0.00 0.00 0.00 Syringe, 10CC, Luer Lock Tip, Becton Dickinson, 309604, 100EA/BX, 4BX/CS 0.00 Tablet, Glucose, Diabetes Care, Becton Dickinson, 328230, 6EA/PK, 12PK/CS 0.00 Blood Collection Set, Safety-Lok, 23G X 3/4" Needle, 12" Tubing w/ Luer Adapter, Becton Dickinson, 367283, 50EA/BX, 4BX/CS Blood Collection Set, Safety-Lok, 25G X 3/4" Needle, 12" Tubing w/ Luer Adapter, Becton Dickinson, 367285, 50EA/BX, 4BX/CS Scalpel, Disposable, Sterile, Stainless Steel, Size 10, Becton Dickinson, 371610, 10EA/BX, 10BX/CS 0.00 0.00 0.00 Scalpel, Disposable, Sterile, Stainless Steel, Size 15, Becton Dickinson, 371615, 10EA/BX, 10BX/CS IV Catheter, Saf-T-Intima, 22G X 3/4" w/ Wings, Y Adapter & Needle Shield, Becton Dickinson, 383323, 25EA/BX, 8BX/CS Infusion Set, E-Z Winged, 25G X 3/4" w/ 12" Tubing, Needle Shield, Becton Dickinson, 387226, 50EA/BX, 4BX/CS 0.00 0.00 0.00 Page 56 Becton Dickinson 387246 120 Becton Dickinson 524030 Thermometer, Digital, Standard, Oral, Becton Dickinson, 524030, EA 305480 NESTABLE SHARPS COLLECTOR 14QT, LG FUNN,CLR,AUTOCL., , BECTON DICKINSON, 305480,20 EA / CS 121 BECTON DICKINSON 122 BECTON DICKINSON 305551 123 BECTON DICKINSON 367298 124 BECTON DICKINSON 305487 125 BECTON DICKINSON 381423 126 BECTON DICKINSON 381433 127 Becton Dickinson 367212 128 Becton Dickinson 305106 129 Becton Dickinson 305903 130 Becton Dickinson Infusion Set, E-Z Winged, 21G X 3/4" w/ 12" Tubing, Needle Shield, Becton Dickinson, 387246, 50EA/BX, 4BX/CS 309570 0.00 0.00 0.00 SHARPS CONTAINER 5.4QT #305551, BECTON DICKINSON, 305551,20EA/CS 0.00 VACUTAINER SFTY-LOK SET 367298, BECTON DICKINSON, 367298,50/BX,4BX/CS 0.00 SHARPS CONTAINER 1.4, BECTON DICKINSON, 305487,36EA/CS 0.00 BD AUTOGUARD IV CATHETER 22G X 1" 999104288, BECTON DICKINSON, 381423,50EA/BX, 4BX/CS BD AUTOGUARD IV CATHETER 20G X 1." 50/ES/BX 4/BX/CS 999104289, BECTON DICKINSON, 381433,50EA/BX, 4BX/CS 0.00 0.00 Blood Collection Needle, Multisample, 21Gx1", Becton Dickinson, 367212, 100/BX 0.00 Needle, Hypodermic, 30Gx1/2", Becton Dickinson, 305106, 100/BX 0.00 Syringe, SafetyGlide, 1ml, 25Gx5/8", Becton Dickinson, 305106, 50/BX 0.00 Syringe, 3cc, 25Gx5/8", Becton Dickinson, 309570, 100/BX 131 0.00 Becton Dickinson 309571 Syringe, 3cc, 23Gx1", Becton Dickinson, 309571, 100/BX 132 0.00 Becton Dickinson 309574 Syringe, 3cc, 22Gx1 1/2", Becton Dickinson, 309574, 100/BX 133 0.00 Becton Dickinson 309581 134 RFP No. 10-475-000-J Medical and Dental Supplies Syringe, 3cc, 25Gx1", Becton Dickinson, 309581, 100/BX, 8BX/CS 0.00 Page 57 Becton Dickinson 309623 135 Becton Dickinson 309626 136 Becton Dickinson 329464 137 BECTON DICKINSON 221854 Syringe, Tuberculin, 1cc, 27Gx1/2", Becton Dickinson, 309623, 100/BX 0.00 Syringe, Tuberculin, 1cc, 25Gx5/8", Becton Dickinson, 309626, 100/BX 0.00 Syringe, Safety Lok, 1cc, 29Gx1/2", Becton Dickinson, 329464, 100/BX 0.00 Agar Plate, Corn Meal, Tw 80, Becton Dickinson,221854,Ct 138 0.00 BECTON DICKINSON 221733 Agar Plate, Cdc Anaer Blood, Becton Dickinson, 221733, 20/Ct 139 0.00 BECTON DICKINSON 221270 140 BECTON DICKINSON 221267 Agar Plate, Macconkey Ii, Becton Dickinson, 221270, 100/Ct 0.00 Agar Plate, Choc Ii, 7W, Becton Dickinson, 221267, 100/Ct 141 0.00 BECTON DICKINSON 309582 Syringe, 3Cc, 25Gx1/5, Becton Dickinson, 309582, 100/Bx 142 0.00 BECTON DICKINSON 381167 Angiocath Iv Cath, 14G, Becton Dickinson, 381167, Ea 143 0.00 BECTON DICKINSON 381137 Angiocath Iv Cath, 20G, Becton Dickinson, 381137, Ea 144 0.00 BECTON DICKINSON 381123 Angiocath Iv Cath, 22G, Becton Dickinson, 381123, Ea 145 0.00 BECTON DICKINSON 381112 Angiocath Iv Cath, 24G, Becton Dickinson, 381112, Ea 146 0.00 BECTON DICKINSON 240952 Bbl Staphyloslide Latex Kit, Becton Dickinson, 240592, Ea 147 0.00 BECTON DICKINSON 371110 148 BECTON DICKINSON 371111 149 BECTON DICKINSON 371115 150 RFP No. 10-475-000-J Medical and Dental Supplies Blade, #10 Sterile Surgeons, Becton Dickinson, 371110, 50/Bx 0.00 Blade, #11 Sterile Surgeons, Becton Dickinson, 371111, 50/Bx 0.00 Blade, #15 Sterile Surgeons, Becton Dickinson, 371115, 50/Bx 0.00 Page 58 BECTON DICKINSON 7251 Blood Collection Set, 21G, Becton Dickinson, 7251, 50/Bx 151 0.00 BECTON DICKINSON 7253 Blood Collection Set, 23G, Becton Dickinson, 7253, 50/Bx 152 0.00 BECTON DICKINSON 7255 Blood Collection Set, 25G, Becton Dickinson, 7255, 50/Bx 153 0.00 BECTON DICKINSON 7210 Blood Collection Set, 22G X 1, Becton Dickinson, 7210, 100/Bx 154 0.00 BECTON DICKINSON 220109 Culturette Ii, Dual St, Becton Dickinson, 220109, 50/Ct 155 0.00 BECTON DICKINSON 207377 Bandage, Elastic 4", Becton Dickinson, 207377, Cs 156 0.00 BECTON DICKINSON 381123 Iv Cath Angiocath, 22Gx1", Becton Dickinson, 381123,Bx 157 0.00 BECTON DICKINSON 305155 Needle, 22Gx1", Becton Dickinson, 305155,100/Bx 158 0.00 BECTON DICKINSON 305145 Needle, 23Gx1", Becton Dickinson, 305145, 100/Bx 159 0.00 BECTON DICKINSON 305122 Needle, 25Gx5/8", Becton Dickinson, 305122, 100/Bx 160 0.00 BECTON DICKINSON 305125 Needle, 25Gx1", Becton Dickinson, 305125, 100/Bx 161 0.00 BECTON DICKINSON 305127 Needle,25Gx1 1/2", Becton Dickinson, 305127, 100/Bx 162 0.00 BECTON DICKINSON 305156 Needle, 22Gx1.5, Becton Dickinson, 305156, 100/Bx 163 0.00 BECTON DICKINSON 305195 Needle, 18Gx1", Becton Dickinson, 305195, 100/Bx 164 0.00 BECTON DICKINSON 305176 165 BECTON DICKINSON 305554 166 RFP No. 10-475-000-J Medical and Dental Supplies Needle, 20Gx1.5", Disposable, Becton Dickinson, 305176, 100/Bx 0.00 Syringe,Safety,Tb,1Cc 25Gx5/8,Becton Dickinson,305554,100/Bx 0.00 Page 59 BECTON DICKINSON 309594 167 BECTON DICKINSON 309603 Syringe, Safety, 3Cc 23X1, Becton Dickinson, 309594, 100/Bx 0.00 Syringe, Luer Lock, 5Cc, Becton Dickinson, 309603, 100/Bx 168 0.00 BECTON DICKINSON 309585 169 BECTON DICKINSON 305553 170 BECTON DICKINSON 36-7253 171 Beiersdorf 231 172 Beiersdorf 1053 173 Beiersdorf 4405 174 BIERSDORF Syringe Only, 3Cc, Luer Lock, Becton Dickinson, 309585, 100/Bx 0.00 Syringe, Safety, 1Cc 27Gx1/2, Tb, Becton Dickinson, 305553, 100/Bx 0.00 Vacutainer, 23G X 3/4, Blue, Becton Dickinson, 36-7253, 50/Bx 0.00 Dressing, Bandage, Fabric, Adhesive, 1" X 3", Latex Free, Beiersdorf, 231, 100EA/BX, 12BX/CS 0.00 Gauze Bandage, Unna Boot, 4" X 10Yd, Beiersdorf, 1053, 12EA/CS 0.00 Brace, Knee, Cotton, Small, 101/2" X 12-1/2", Rayon Elastic, Beiersdorf, 4405, EA 4505 Ankle Brace, Large, #45, 4505, Ea DE1070-A Electrode, Resting Tab, AG/AGCL, Bio-Detek Inc., DE1070-A, 100EA/BG, 5BG/BX, 10BX/CS 0.00 175 0.00 Bio-Detek Inc. 176 BIOSYS LABS, INC U39 0.00 Uriscan Strips, 10Sg, Biosys Labs, Inc, U39, 100/Ct 177 0.00 Biosys Labs, Inc. 25 178 BOEHRINGER MANN 911 Test Strip, Urine, w/ General Gloves, Biosys Labs, Inc., 25, 100EA/VL, 1VL/BX, 10BX/CS 0.00 A/C Instantplus Cholesterol, Mann, 911, 25/Bx 179 0.00 BOWMAN MFG 1500 Glove Dispenser, Bowman Mfg, 1500, Ea 180 0.00 BRANDT INDUSTRIES 70002 181 BRECKENRIDGE INC. 51736 182 RFP No. 10-475-000-J Medical and Dental Supplies Screen, Privacy Folding, 4 Panel, Brandt Industries, 70002, Ea 0.00 TRIPLE ANTIBIOTIC IND PK 1GM, BRECKENRIDGE INC., 51736,144EA/BX, 12BX/CS 0.00 Page 60 Brewer 0985-5001-04 Table, Exam, w/ Pelvic Tilt, w/ Drawer Heater, Burgundy, Brewer, 0985-5001-04, EA 0730-1307 Dressing, Bandage, Fabric, Adhesive, Large Finger Tip, Latex Free, BSN-Jobst, 07301307, 50EA/BX, 12BX/CS 183 BSN-Jobst 184 Burdick 47029 185 BURDICK 1055 0.00 0.00 Sensors, Disposable, CardioSense/Ultra II, Burdick, 47029, 500EA/BX, 10BX/CS 0.00 Bp Cuff, Large, Burdick, 1055, Ea 186 0.00 BURNSHINE B9 Cleaner Enzmatic, B9, Gal/Bx 187 0.00 BURNSHINE S5 Instrument Germicide, S5, 188 0.00 Burton Medical Products Corp. 224112 189 Busse 142 190 Busse 202 191 Busse 297 Light, Outpatient II, w/ Fleximount Floor Stand, Burton Medical Products Corp., 224112, EA 0.00 Syringe, Sterile, 2OZ, Ear/Ulcer, Disposable, Latex Free, Busse, 142, 50EA/CS 0.00 Gown, Isolation, Non-Sterile, Latex Free, Yellow, Busse, 202, 50EA/CS 0.00 Suction Tip, Rigid Open, Sterile, w/ Vent, Busse, 297, 50EA/CS 192 0.00 Busse 346 193 Busse 348 194 Busse 490 195 Busse 715 Cover, Shoe, Sur-Step, Bulk, w/ Anti-Skip Strips, Universal Size, Busse, 346, 150PR/CS 0.00 Cover, Shoe, Non-Conducting, Universal, Busse, 348, 50PR/BX, 3BX/CS 0.00 Cup, Denture, w/ Engraved, Turquoise Lid, Plastic, Busse, 490, 250EA/CS 0.00 Kit, Tracheostomy Care, Sterile, Busse, 715, 1KIT/BX, 20BX/CS 196 0.00 Busse 716 Kit, Skin Staple Remover, Stainless Steel, Sterile, Busse, 716, 48EA/CS 723 Kit, Suture Removal, Sterile, 1 Iris Scissors, Straight, 4-1/2", 1 Adson Serrated Forceps, 4-3/4", 1 Large Alcohol Prep Pad, 1 197 Busse 198 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 Page 61 Idaho PVP Prep Pad, and 1 3" x 3" 12Ply Gauze Dressing, Latex Free, Busse, 723, 50EA/CS Busse 737 Kit, Suture Removal, Sterile, Littauer Tip, Busse, 737, 48EA/CS 747 Kit, Suturing, 1 Halsey Needle Holder, Fine Point, 5", 1 Iris Scissors, Straight, 1 Adson Forceps, 2 2oz Medicine Cups, 5 2" x 2" Gauze Sponges, 8 4" x 4" Gauze Sponges, 1 18" x 26" Fenestrated Drape, 1 Absorbent Towel, Blue, Busse, 747, 20EA/CS 199 Busse 200 Busse 749 Tray, Laceration, ER, w/ Floor Grade Instruments, Busse, 749, 20EA/CS 800 Set, Tracheostomy Care, Sterile, w/ Hydrogen Peroxide, 1 Pair of Gloves, 1 Polylined Drape, Busse, 800, 20EA/CS 201 Busse 202 Busse 821 0.00 0.00 0.00 0.00 IV Start Kit, w/ Tegaderm Dressing, Busse, 821, 50EA/CS 203 0.00 BUSSE 696 Drape, 18 X 26 Plain, Busse, 696, 50Bx 204 0.00 BUSSE 759 Dressing Change Tray, Busse, 759, Ea 205 0.00 BUSSE 1124 Infection Control Kit Deluxe, Busse, 1124, Ea 206 0.00 BUSSE 751 Minor Laceration Pack, Busse, 751, Ct 207 0.00 C&P Healthcare Corp 59950 MOORE/4910 208 CALTECH INDUSTRIES INC 23032 Bandage, Triangular, NonSterile, 36" X 30" X 51", C&P Healthcare Corp, 59950 MOORE/4910, 12EA/BX 0.00 Cleaner, Precise QTB, Caltech Industries Inc, 23032, 32Oz 209 0.00 CAN-AM CARE 810114 Lancets, Can-Am Care, 810114, Bx 210 0.00 Cardinal Health 001811 211 RFP No. 10-475-000-J Medical and Dental Supplies Tubing, Connector, Two Oxygen Tubes, Cardinal Health, 001811, 50EA/CS 0.00 Page 62 Cardinal Health 002006 212 Cardinal Health 002034 213 Cardinal Health 3274 214 Cardinal Health 1472950 215 Cardinal Health T16/15 Humidifier, Empty, 6PSI, Blue, Cardinal Health, 002006, 50EA/CS 0.00 Nebulizer, Medicinal, Tee Adapter, 7' Tubing, Cardinal Health, 002034, 50EA/CS 0.00 Cap, Bouffant, Spunbound, Blue, Cardinal Health, 3274, 750EA/CS 0.00 Ointment, Zinc Oxide, USP, 1OZ Tube, Cardinal Health, 1472950, 1EA/PK, 6PK/BX 0.00 Device, Biopsy, Temno, Cardinal Health, T16/15, 5EA/CS 216 0.00 CARDINAL HEALTH 001301 217 CARDINAL HEALTHCARE 8848A 218 CARDINAL HEALTHCARE 8847A 219 CARDINAL HEALTHCARE 8888I TUBING OXYGEN 7' VINYL TIPPED, CARDINAL HEALTH, 001301,50EA/CS 0.00 Glove, Exam N/S Latex Textured Pf, Instaguard, Cardinal Health, Cs 0.00 Glove, Exam N/S Latex Textured Pf, Instaguard, Cardinal Health, Cs 0.00 Glove, Instaguard Pf Exam, Cardinal Healthcare, 8888I, Cs 220 0.00 CARDINAL HEALTHCARE 8887I Glove , Instaguard Pf Exam, Cardinal Healthcare, 8887I, Cs 221 0.00 CARDINAL HEALTHCARE 8886I Glove, Instaguard Pf Exam, Cardinal Healthcare, 8886I, Cs 222 0.00 CHASE SCIENTIFIC 7101 Microscope Slides, Plain, Chase Scientific, 7101, Bx 223 0.00 Chester Labs 535-4-96 224 Rinse, Mouth, Alcohol, Blue, 4OZ, Chester Labs, 535-4-96, 96EA/CS 0.00 560-5.8 Scrub, Alcohol Foam, 5.8OZ, Chester Labs, 560-5.8, 24EA/CS D11000 226 Test Strip, Reagent, Full Urinalysis, DiaScreen 10, D11000, 100EA/VL, 1VL/BX, 10BX/CS 0.00 D11200 227 Test Strip, Reagent, Urinalysis, Screens for Glucose & Protein, D11200, 100EA/VL, 1VL/BX, 10BX/CS 0.00 Chester Labs 225 0.00 Chronimed Inc. Chronimed Inc. RFP No. 10-475-000-J Medical and Dental Supplies Page 63 Chronimed Inc. D11900 228 Chronimed Inc. D13400 229 Clark Pillow Company SSC-116 230 CLINTON M-22 Test Strip, Reagent, Urinalysis, DiaScreen 9, D11900, 100EA/VL, 1VL/BX, 10BX/CS 0.00 Test Strip, Reagent, Urinalysis, DiaScreen 4NL, D13400, 100EA/VL, 1VL/BX, 10BX/CS 0.00 Pillow, Moisture Proof, White, 17" X 24", 16OZ Fill, Clark Pillow Company, SSC-116, 16EA/CS 0.00 Mayo Stand, Clinton, M-22, Ea 231 0.00 Coloplast Corporation 2650 Paste, Barrier, 2OZ, Coloplast Corporation, 2650, 12EA/CS 232 0.00 Connecticut Clean Room Corp NON 24556 233 CONSOLIDATED PRODUCTS 4299.1 234 Conva Tec 187955 Cover, Shoe, Regular, Blue, Polyethylene, Connecticut Clean Room Corp., NON 24556, 50PR/BG, 10BG/CS 0.00 Instant Cold Compress, Consolidated Products, 4299.1, Cs 0.00 Dressing, Extra Thin, 4' x 4", Conva Tec, 187955, 10EA/BX 235 0.00 Conva Tec 324908 Moisture Barrier, Protective, Conva Tec, 324908, 12EA/CS 236 0.00 Conva Tec 651031 Dressing, Combiderm, 4" X 4", Conva Tec, 651031, 10EA/BX 71430 Sunscreen, Pouch, SPF 30+, Dispenser Shell Clam, CoreTex Products, 71430, 25EA/BG, 8BG/CS 237 0.00 CoreTex Products 238 Cosco Enterprise 01544-16 0.00 Soap, Green Tincture, Cosco Enterprise, 01544-16, EA 239 0.00 Cramer Products, Inc. 32746 240 Cramer Products, Inc. 32846 241 Cumberland-Swan 87143 242 Custom Labs TG30006 243 RFP No. 10-475-000-J Medical and Dental Supplies Pack, Cold, Reusable, Regular, 6" X 9", Flex-I-Cold, Cramer Products, Inc., 32746, 12EA/CS 0.00 Pack, Cold, Reusable, Small, 4" X 6", Flex-I-Cold, Cramer Products, Inc., 32846, 12EA/CS 0.00 Peroxide, Hydrogen, 3% Solution, 16OZ, CumberlandSwan, 87143, 12EA/CS 0.00 Glucose, Trutol, Orange, Sundex, 100G, Custom Labs, TG30006, 12EA/CS 0.00 Page 64 CYPRSS 25-92 Glove,Sm Vinyl Pf, 25-92,100/Cs 244 0.00 Dey L.P. 50001 EpiPen, (1 PK) 0.3MG, Dey L.P., 50001, 1EA/BX, 12BX/CS 245 0.00 Dey L.P. 50101 EpiPen, Jr, (1 PK) 0.15MG, Dey L.P., 50101, 1EA/BX, 12BX/CS 246 0.00 Dey L.P. 0500-02 247 Dial Corporation 80733 248 Dial Corporation 84019 249 Dial Corporation 84014 EpiPen, 2 Pack (2 Pens + Trainer), 0.3MG, Dey L.P., 050002, 2EA/BX, 6BX/CS 0.00 Soap, Liquid Antimicrobial, 1.5OZ, Dial Corporation, 80733, 72EA/CS 0.00 Soap, Liquid Antimicrobial, Liter Refill, Dial Corporation, 84019, 8EA/CS 0.00 Soap W/Pump, Liquid, 7.5oz, Dial, 84014, 1EA 250 0.00 DONOVAN IND HB01 Hairbrush, Standard Bristles, Donovan , Hb01, Ea 251 0.00 DONOVAN IND TNC3282 Toe Nail Clipper With File, Donavon, Tnc3282, Cs 252 0.00 Donovan Industries Inc FNC3268 253 Drug Free Enterprises 60505 254 DUKAL 44122 Clipper, Nail, Finger, w/ File, Donovan Industries Inc, FNC3268, 6EA/BX, 48BX/CS 0.00 Test, 5 Panel Cup, Drug Check w/ PCP, Drug Free Enterprises, 60505, 25EA/BX 0.00 Gauze, Poly, 2X2, 4Ply, N/S, Dukal Corp, 44122, Ct 255 0.00 DUKAL CORP 503 Bandage, Elastic 3 X 5", Dukal Corporation, 503, 10/Bx 256 0.00 DUKAL CORP 4084IM Gauze, 4X4 8-Ply N/S, Dukal Corporation, 4084Im, 200/Bx 257 0.00 DUKAL CORP 12276 Gauze Sponge, 2X2 12 Ply, Dukal, 12276, Ct 258 0.00 Dukal Corporation P110 259 RFP No. 10-475-000-J Medical and Dental Supplies Tape, Paper, Hypoallergenic, 1" X 10Yd, Dukal Corporation, P110, 12RL/BX, 12BX/CS 0.00 Page 65 DUKAL CORPORATION 44123 260 DUKAL CORPORATION M49252 POLY GAUZE 3X3 4 PLY NS, DUKAL CORPORATION, 44123,200EA/BG, 20BG/CS 0.00 Bandage, Conform, 4 X 4.1 Yds, Ns, Dukal Corporation, M49252 261 0.00 DUKAL CORPORATION 6412 Gauze, 4X4 12Ply Sterile, Dukal Corporation, 6412, 50/Bx 262 0.00 DUKAL CORPORATION 8250 Gauze Pads, 2X2 Sterile, Dukal, 8250, Bx 263 0.00 DUKAL CORPORATION 37336 Gauze Sponge, 2X2, 8 Ply Ns, Dukal 37336, Ct 264 0.00 DUKAL CORPORATION 3233 Gauze Sponge, 3X3 12Ply, Dukal, 3233, Ct 265 0.00 DUKAL CORPORATION 12277 Gauze Sponge, 3X3 12Ply, Dukal, 12277, Ct 266 0.00 DUKAL CORPORATION 12771 Gauze Sponge, 3X3 8 Ply, Dukal 12771, Ct 267 0.00 DUKAL CORPORATION 12279 Gauze Sponge, 4X4 12 Ply, Dukal, 12279, Ct 268 0.00 DUKAL CORPORATION 12278 Gauze Sponge , 4X4 8Ply, Dukal, 12278, Ct 269 0.00 DUKAL CORPORATION 4122IM Gauze Sponge, 4X4 N/S, Dukal, 4122Im, Bx 270 0.00 DYNAREX B67001 Alcohol Prep Pad, Dynarex, B67001, 100/Bx 271 0.00 DYNAREX 1311 Wipes, Baby, Dynarex Corp, 1311, 272 0.00 1401 Ammonia Inhalant, 0.33CC, Dynarex Corporation, 1401, 10EA/BX 3322 274 Gauze Sponge, Surgical, Sterile, 2" X 2", 8Ply, Dynarex Corporation, 3322, 2EA/ENV, 50ENV/BX, 30BX/CS 0.00 3343 275 Gauze Sponge, Surgical, Sterile, 4" x 4", 12Ply, Dynarex Corporation, 3343, 2EA/ENV, 25ENV/BX, 24BX/CS 0.00 Dynarex Corporation 273 Dynarex Corporation Dynarex Corporation RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 66 3601 276 Bandage, Adhesive, Strip, Sheer, Sterile, 3/4" X 3", Dynarex Corporation, 3601, 100EA/BX, 24BX/CS 0.00 3608 277 Bandage, Adhesive, Strip, Sheer, Sterile, 3/8" X 3", Junior, Dynarex Corporation, 3608, 100EA/BX, 36BX/CS 0.00 3614 278 Bandage, Adhesive, Fabric, Strip, Sterile, 2" X 4-1/2", Dynarex Corporation, 3614, 50EA/BX, 24BX/CS 0.00 3617 279 Bandage, Adhesive, Fabric, Strip, Sterile, 1-3/4" X 2", Fingertip, Dynarex Corporation, 3617, 100EA/BX, 24BX/CS 0.00 Dynarex Corporation Dynarex Corporation Dynarex Corporation Dynarex Corporation Dynarex Corporation B67003 Pad, Alcohol Prep, Medium, 1" X 2.5", Dynarex Corporation, B67003, 100EA/BX, 20BX/CS P-D41900 Castile Soap, Towelette, w/o BZK, w/ 2% Coconut Oil, Dynarex Corporation, P-D41900, 1EA/PK, 100PK/BX, 10BX/CS 280 Dynarex Corporation 281 Dynarex Corporation 3614 0.00 0.00 Bandages, Fabric Strip, X-large 2"x4", Dynarex, 3614, 50/BX 282 0.00 Dynarex Corporation 3223 283 Dynarex Corporation 3354 Gauze Sponge 2"x2", 12-ply, NonSterile, Dynarex, 3223, 200/BX, 8000/CS 0.00 Gauze Pads 4"x4", 12-Ply Sterile, Dynarex, 3354, 100/BX 284 0.00 Dynarex Corporation 3572 Tape, Transparent, 1", Dynarex, 3572, 12/BX 285 0.00 Dynarex Corporation 3602 Bandage ,Plastic, 1"x3", Dynarex, 3602, 100/BX 286 0.00 Dynarex Corporation 3611 Bandage, Fabric, 3/4"x3", Dynarex, 3611, 100/BX 287 0.00 Dynarex Corporation 3612 Bandage, Fabric, 1"x3", Dynarex, 3612, 100/BX 288 0.00 Dynarex Corporation 2329 289 Dynarex Corporation 3607 Surgical Gloves, Latex, LightlyPowdered, X-large, Dynarex, 2329, 100/BX 0.00 Spot Bandage, 7/8", Dynarex, 3607, 100/BX 290 0.00 DYNAREX CORPORATION D70600 Wipes, Bacterial, Dynarex, D70600, 100/Bx 291 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 67 DYNAREX CORPORATION 3710 Cotton Balls, Medium, Dynarex Corporation, 3710, 2000/Ct 292 0.00 E Fougera 23504 293 EMPLOY+ABILITY 4298 Ointment, Vitamins A & D, 4OZ Tube, E Fougera, 23504, 6EA/BX 0.00 Instant Hot Compress, Employ+Ability, 4298, Ct 294 0.00 EMPLY+ABILITY 4306 Cold/Hot Reusable Gel Pack, Employ+Ability, 4306, Ea 295 0.00 Encompass/HospitexLintex 46583-113 296 Tunic, Square Neck, Ceil Blue, Small, Encompass/HospitexLintex, 46583-113, EA 0.00 46583-115 Tunic, Square Neck, Ceil Blue, Medium, Encompass/HospitexLintex, 46583-115, EA 47974-605 298 BATH TOWEL ALL-COT 20X40 5LB, ENCOMPASS/HOSPITEXLINTEX, 47974605,12EA(DZ)BX 0.00 299 Gown, Exam, Patient, Tissue/Poly/Tissue, 30" X 42", White, Stretchable Waist Ties, Encore, 9810846, 50EA/CS 0.00 Encompass/HospitexLintex 297 ENCOMPASS/HOSPITEXLINTEX Encore ETHICON 9810846 2013 NON-ADHERING DRESSING 3X8", ETHICON, 2013,36EA/BX,6BX/CS 26026 Syringe, 1/2CC, Insulin w/ Needle, 28G X 1/2", Retail Pack, Latex Free, Exel Corporation, 26026, 10EA/BG, 10BG/BX, 5BX/CS 300 Exel Corporation 301 E-Z-EM 816 0.00 0.00 0.00 Enema Bag, 16OZ w/ Barium, EZ-EM, 816, 24EA/CS 302 0.00 E-Z-EM L196 303 FASTMARK INC FASTAID JR Liquid, 12OZ, E-Z Paque, 60% W/U w/ Caps, E-Z-EM, L196, 24EA/CS 0.00 Fastaid First Aid Guide, Fastmark,Inc, Fastaid Jr, Ea 304 0.00 IB-013 305 Brief, Adult, Full Mat, Super Absorbent, Large, First Quality Products Inc, IB-013, 12EA/BG, 6BG/CS 0.00 IB-014 306 Brief, Adult, Full Mat, Super Absorbent, X-Large, First Quality Products Inc, IB-014, 16EA/BG, 4BG/CS 0.00 First Quality Products Inc First Quality Products Inc RFP No. 10-475-000-J Medical and Dental Supplies Page 68 First Quality Products Inc IBF-016 307 First Quality Products Inc NU-014 Brief, Adult, Nu-Fit, X-Large, First Quality Products Inc, NU014, 10EA/BG, 6BG/CS UP-100 Under pad, Super, Spun bond, Peach, 30" X 30", First Quality Products Inc, UP-100, 10EA/BG, 10BG/CS 308 First Quality Products Inc 309 First Quality Products Inc WW-701 310 First Quality Products Inc PV-513 311 Fisher 7118-22278705 312 Graham Field 95-3719 313 GRAHAM FIELD Brief, Adult, Full Mat, Frontal Tape, Regular, Disposable, First Quality Products Inc, IBF-016, 10EA/BG, 8BG/CS 47-1590 0.00 0.00 0.00 Wipe, Wet, Disposable, First Quality Products Inc, WW-701, 48EA/TUB, 1TUB/BX, 12BX/CS 0.00 Brief - Protective Underwear, Large, First Quality Corporation, PV513, 72/CS 0.00 Blood Collection Device, Safety, Fisher, 7118-22278705, 250EA/PK, 4PK/CS 0.00 Paper Lens, 4" X 6", Graham Field, 95-3719, 50SHTS/PK, 12PK/BX 0.00 Applicator, 6", Silver Nitrate, Graham Field, 47-1590, 100/Bx 314 0.00 GRAHAM FIELD 70221N 315 GRAHAM FIELD 70180N 316 GRAHAM FIELD 70170N 317 GRAHAM FIELD 70002N Exam Gown, 30X42 3 Ply White, Graham Medical Products, 70221N, Cs 0.00 Towel, Plasback, 13 1/2, 3Ply, White, Graham Medical, 70180N, Cs 0.00 Towel, Professional, 13 1/2, 3Ply, White, Graham Medical, 70170N, Cs 0.00 Table Paper, 18" White Crepe, Graham Medical, 70002N, Cs 318 0.00 GRAHAM FIELD 70016N Table Paper, 18" White Smooth, Graham Medical, 70016N, Cs 319 0.00 GRAHAM FIELD 70004N Table Paper, 21" White Crepe, Graham Medical, 70004N, Cs 320 0.00 GRAHAM FIELD 70018N Table Paper, 21" White Smooth, Graham Medical, 70018N, Cs 321 0.00 GRAHAM MEDICAL PRODUCTS 70300N 322 RFP No. 10-475-000-J Medical and Dental Supplies DRAPE SHEET 40X48 2PLY WHT 300, GRAHAM MEDICAL PRODUCTS, 70300N,100EA/CS 0.00 Page 69 H&P 2448861 Alcohol Gel, 40Z, H&P, 2448861, Bx/Ea 323 0.00 H&P 1753439 Baby Wipes, Freshette, H&P, 1753439, 720/Cs 324 0.00 H&P 1278624 Wipes, Triad, Gentle Relief, H&P, 1278624, Bx 325 0.00 Hartman Conco 193045 326 Hartman Conco 16200025 327 Bandage, Elastic, Sterile, 3" X 4.1Yd, Hartman Conco, 193045, 12EA/BX, 8BX/CS 0.00 Bandage, Elastic, Reinforced, 2" X 5Yd, Hartman Conco, 16200025, 10EA/BX, 6BX/CS 0.00 16300025 Bandage, Elastic, Reinforced, 3" X 5Yd, Hartman Conco, 16300025, 10EA/BX, 6BX/CS 19200000 329 Bandage, Elastic, Latex Free, Sterile, 2" X 4.1Yd, Hartman Conco, 19200000, 12EA/BX, 8BX/CS 0.00 33390000 330 Bandage, Elastic, Latex Free, Sterile, 3" X 4.5Yd, Hartman Conco, 33390000, 10EA/BX, 6BX/CS 0.00 Hartman Conco 328 Hartman Conco Hartman Conco Hartmann Conco 33490000 331 0.00 Bandage, Elastic, Flesh, 4" X 4.5Yd, Hartman Conco, 33490000, 10EA/BX, 6BX/CS 0.00 33690000 Bandage, Elastic, Flesh, 6" X 4.5Yd, Hartman Conco, 33690000, 10EA/BX, 6BX/CS 59540000 333 Bandage, Latex Free, Self Closure, 4" X 5Yd, Hartman Conco, 59540000, 10EA/BX, 6BX/CS 0.00 59560000 334 Bandage, Latex Free, Self Closure, 6" X 5Yd, Hartman Conco, 59560000, 10EA/BX, 6BX/CS 0.00 80100000 335 Bandage, Elastic, Latex Free, Non-Sterile, 1" X 4.1Yd, Hartman Conco, 80100000, 24EA/BX, 4BX/CS 0.00 80200025 336 Bandage, Elastic, Latex Free, Non-Sterile, 2" X 4.1Yd, Hartman Conco, 80200025, 12EA/BG, 8BG/CS 0.00 80300000 337 Bandage, Elastic, Latex Free, Non-Sterile, 3" X 4.1Yd, Hartman Conco, 80300000, 12EA/BG, 8BG/CS 0.00 80400000 338 Bandage, Elastic, Latex Free, Non-Sterile, 4" X 4.1Yd, Hartman Conco, 80400000, 12EA/BG, 8BG/CS 0.00 Hartman Conco 332 Hartman Conco Hartman Conco Hartman Conco Hartman Conco Hartman Conco Hartman Conco RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 70 Hartman Conco 81300025 339 HARTMAN CONCO 33290000 Bandage, Elastic, Latex Free, Sterile, 3" X 4.1Yd, Hartman Conco, 81300025, 12EA/BG, 8BG/CS 0.00 Bandage, Elastic 2", Hartman Conco, 33290000, Ea 340 0.00 HARTMAN CONCO 16400025 Bandage, Elastic 4", Hartman Conco, 16400025, Bx 341 0.00 HARTMAN CONCO Flexicon, Sterile Gauze 4 X 4.1 Yds, Hartman Conco, 19400000, Bx Hausmann Industries, Inc Couch, w/ Cabinet & Sliding Doors, 72" X 27" X 22", Hausmann Industries, Inc., 7030, 6PARTS/COUNCHUNIT, 1 COUCHUNIT/BX 342 7030 343 HEALTHLINK 7720 0.00 0.00 Soap, Aloeguard 800Ml, Healthlink, 7720 344 0.00 HEALTHLINK 7740 Soap, Aloeguard, Healthlink, 7740, Gal/Bx 345 0.00 HEATLHLINK 7135 Disinfectant, Citrofoam, Healthlink, 7135, Ea/Bx 346 0.00 Hermitage Hospital Products 1801 347 Hermitage Hospital Products 1902 348 HERMITAGE HOSPITAL PRODUCTS 69616 349 HERMITAGE HOSPITAL PRODUCTS 69618 350 Hollister 17121 Blade, Tongue, Regular, 6" X 3/4", Non-Sterile, Hermitage Hospital Products, 1801, 50EA/BX, 10BX/CS Applicator, Cotton Tipped, Sterile, Wooden Shaft, 6", Hermitage Hospital Products, 1902, 2EA/BG, 100BG/BX, 10BX/CS WOOD TONGUE BLADES JR NS MMC, HERMITAGE HOSPITAL PRODUCTS, 69616,500EA/BX, 10BX/CS 0.00 0.00 0.00 Tongue Blades, Wood, Adult, Sterile, Hermitage Hospital, 69618, Bx 0.00 Hygiene Kit, Pump, Hand, Sterile, Hollister, 17121, EA 351 0.00 Hollister 17126 352 Hollister 17131 Hygiene Kit, Pump, Hand, Dual, w/ Flex Shield, Sterile, Hollister, 17126, EA 0.00 Hygiene Kit, Dual, Sterile, Hollister, 17131, EA 353 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 71 Hormel Healthlabs Inc 27016 354 Supplement, Liquid for Malnutrition, Medium Pass Vani32, Hormel Healthlabs Inc, 27016, 12EA/CS 0.00 14147 Napkin, Sanitary, Feminine Hygiene, Disposable, Hospital Specialty Co, 14147, 250EA/CS 1041 356 Mask, Concentration, Medium, Elongated w/ 7' Tubing, Hudson Respiratory Care, Inc., 1041, 50EA/CS 0.00 1882 357 Nebulizer, Micro Mist w/ Tee Mouthpiece & Small Tubing, Hudson Respiratory Care, Inc., 1882, 50EA/CS 0.00 Hospital Specialty Co 355 Hudson Respiratory Care, Inc. Hudson Respiratory Care, Inc. HUNTLEIGH HEALTHCARE INC BD30000 0.00 Fetal Monitor, Huntleigh Healthcare, Bd3000, Ea 358 0.00 HUNTLEIGH HEALTHCARE, INC. D920-P 359 HUNTLEIGH HEALTHCARE, INC. FD2-OP3 360 I C N Pharmaceuticals 187074631 361 INNOVATIVE HEALTHCARE 162100 362 INNOVATIVE HEALTHCARE 162200 363 INNOVATIVE HEALTHCARE 162300 364 JAMES ALEXANDER CORP 33101-HLM FETAL DOPPLEX D920, HUNTLEIGH HEALTHCARE, INC., D920-P,EA 0.00 FETAL DOPPLEX II 3MHZ PROBE, HUNTLEIGH HEALTHCARE, INC., FD2OP3,EA 0.00 Insta Glucose, 31GM Tube, I C N Pharmaceuticals, 187074631, 12EA/BX, 8BX/CS 0.00 GLOVE EXAM VINYL SYN PF SML, INNOVATIVE HEALTHCARE, 162100,100EA/BX, 10BX/CS 0.00 GLOVE MED VINYL P/F, INNOVATIVE HEALTHCARE, 162200,100EA/BX, 10BX/CS 0.00 GLOVE MED VINYL W/PW, INNOVATIVE HEALTHCARE, 162300,100EA/BX, 10BX/CS 0.00 Ammonia, Aromatic, James Alexander, 33101-Hlm, Bx 365 0.00 JOHNSON & JOHNSON DHV12 DERMABOND DHV12, JOHNSON & JOHNSON, DHV12,12 EA / BX IV0013Y IV Administration Set, w/ Injection Site, Latex Free, 60GTTS, Kawasumi Labs America, Inc., IV0013Y, 50EA/CS 366 Kawasumi Labs America, Inc. 367 Kawasumi Labs America, Inc. IV009Y 368 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 IV Administration Set, w/ Injection Site, Latex Free, 10GTTS, IV009Y, 50EA/CS 0.00 Page 72 Kendall 1020 369 Kendall 2242 370 Kendall Gauze Bandage, Stretch, NonSterile, 3" X 75", Kendall, 2244, 12RL/BG, 8BG/CS 14000 Container, Specimen, Graduated, w/ Metal Screw on Cap, 7OZ, Kendall, 14000, 100EA/CS 372 Kendall 1522SA 373 Kendall 2132-2841 0.00 Gauze Bandage, Stretch, NonSterile, 2" X 75", Kendall, 2242, 12RL/BG, 8BG/CS 2244 371 Kendall Solution, Sterile Bottle, 100ML for Irrigation & Suction, 3.5OZ, Kendall, 1020, 48EA/CS 0.00 0.00 0.00 Collector, Sharps, Red w/ Clear Top, 2.2QT, Kendall, 1522SA, 60EA/CS 0.00 Eye Pad, Curity, Kendall, 21322841, 50EA/BX, 12BX/CS 374 0.00 Kendall 2236 Gauze Roll, Conforming, Sterile, 4"x4.1Yds, Kendall, 2236, 12/BX 375 0.00 Kendall 5110 376 Kendall 6017 Alcohol Prep Pad, Sterile, 2-Ply, Large, Kendall, 5110, 200/BX, 4000/CS 0.00 Telfa Adhesive Pad 2"x3", Kendall, 6017, 100/BX 377 0.00 Kendall 6715 Gauze Roll, Kerlix, 4.5"x4.1Yds, Sterile, Kendall, 6715, 100/CS 378 0.00 Kendall 442214 379 Kendall 8939 Gauze Sponge, Dermacea, Sterile, 4"x4", 12-Ply, 10 per tray, Kendall, 442214, 1280/CS 0.00 Chemo Container w/Lid, 18Gallon, Kendall 8939, 5/CS 380 0.00 Kendall 1180127012 381 Kendall 1180325058 Syringe, Tuberculin, 1cc, 27Gx11/2", Kendall, 1180127012, 100/BX 0.00 Syringe, 3cc, 25Gx5/8", Kendall, 1180325058, 100/BX 382 0.00 Kendall 1180325100 Syringe, 3cc, 25Gx1", Kendall, 1180325100, 100/BX 383 0.00 Kendall 8881511110 384 RFP No. 10-475-000-J Medical and Dental Supplies Syringe, Insulin, Safety, 1cc, 29Gx1/2", Kendall, 8881511110, 100/BX 0.00 Page 73 Kendall 8881511201 385 Kendall 8881601101 Syringe, Safety, Tuberculin,1cc, 28Gx1/2", Kendall, 8881511201, 500/CS 0.00 Syringe, Insulin, 1cc, 28Gx1/2", Kendall, 8881601101, 100/BX 386 0.00 Kendall 8881602018 387 Kendall 8881676285 388 Kendall Probe Covers, Unique to Filac F-1500, Kendall, 8884221000, 500/BX 504000 Thermometer, Digital Filac, F3000 with Oral Probe, Kendall, 1EA (replacement to discontinued F-1500 model) 390 0.00 Sharps Collection Container, 8Quart, Chimney Top, Red, Kendall, 8881676285, 20/CS 8884221000 389 Kendall Lancet, Sterile, 3.2mm puncture depth, 21gauge, Blue, Kendall, 8881602018, 200/BX, 5000/CS 0.00 0.00 0.00 202020 Probe Covers, Unique to Filac F-3000, Kendall, 8884221000, 500/BX 8881302015 392 Blood Collection Tube, Serum Separator, 16mmx100mm, 9ml Draw, Glass, Kendall, 8881302015, 100/BX 0.00 8881302106 393 Blood Collection Tube, Serum Separator, 13mmx100mm, 6ml Draw, Glass, Kendall, 8881302106, 100/BX 0.00 8881311446 394 Blood Collection Tube, Lavender Top, EDTA Additive, 13mmx75mm, 5ml draw, Glass, Kendall, 8881311446, 100/BX 0.00 8881301512 395 Blood Collection Tube, Red Top, No additive, 13mmx100mm, 7ml draw, Glass, Kendall, 8881301512,100/BX 0.00 1903 396 Gauze Sponge, Curity, 3x3, 12Ply Sterile, 2 pads per pack, Kendall, 1903, 50/TRAY, 2400/CS 0.00 Kendall 391 Kendall Kendall Kendall Kendall Kendall Kendall 2600 0.00 Prepping Ball, Curity, Medium, Kendall, 2600, 500/BX, 4000/CS 397 0.00 Kendall 7772 398 Kendall 8881501210 Gauze Sponge, Curex , Sterile, 4x4, 8-Ply, Kendall, 7772, 1200/CS 0.00 Syringe, Insulin, 1cc, 28Gx1/2", Kendall, 8881501210, 500/CS 399 0.00 KENDALL 2247 Bandage, Conform 4", Ns, Kendall, 2247, 12/Ct 400 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 74 KENDALL 8509SA Sharps Container, Disposable, 5 Qt, Kendall, 8509Sa, 20/Cs 401 0.00 KENDALL 8900SA Sharps Disp, Sage, 1Qt, Kendall, 8900Sa, Ea 402 0.00 KENDALL 8970 Sharps Disp, Sage, 2 Gal, Kendall, 8970, Ea 403 0.00 KENDALL 8509SA Sharps Disp, Sage 5 Qt, Kendall, 8509Sa, Ea 404 0.00 KENDALL 8556-H Sharps Disp, Sage, In Room, Kendall, 8556-H, Ea 405 0.00 KENDALL 500-1000-600 Cups, Specimen, 4 1/2 Oz, Ns, Kendall, 500-1000-600, Cs 406 0.00 KENDALL 6818 Alcohol Prep Pads, Webcol, Kendall, 6818, Bx 407 0.00 Kimberly Clark 21400 408 KIMBERLY CLARK 34155 409 Kimberly Clark 96124 Tissue, Facial, White, Kimberly Clark, 21400, 100EA/BX, 36BX/CS 0.00 KIM WIPES, KIMBERLY CLARK, 34155,1 CT / BX, 60 BX / CS 0.00 Isolation Gown, Yellow, Kimberly Clark, 69124, 10/BX 410 0.00 Laerdal Medical Corp. 50010 411 Laerdal Medical Corp. 820011 412 LifeScan 020-244 413 MABIS 123-610-000 Resuscitator, Baby Anne, Four Pack of Mannequins, Laerdal Medical Corp., 50010, 1EA/BX 0.00 Mask, Pocket w/ One Way Valve Filter, Yellow, Laerdal Medical Corp., 820011, EA 0.00 Test Strip, Blood Glucose, One Touch, Monitoring, LifeScan, 020-244, 50EA/BX, 24BX/CS 0.00 Thermometer, Digital, Mabis Healthcare, 123-610-000, Ea 414 0.00 MABIS 123-618-000 Thermometer Probe Covers, Digital, Mabis,123-618-000,Bx 415 0.00 MABIS 4109 Tourniquet, 1In, S/P, Disp, Latex, Mabis, 04109, Ct 416 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 75 MABIS HEALTHCARE 39-902-000 Safety Pins, Sz Med, Mabis Healthcare, 39-902-000 417 0.00 Mabis Healthcare Inc 15-633-000 418 419 MAJOR PHARMACEUTICALS, INC. MALLCKRODT 115923 P-000725-00 Probe Cover, for Oral Thermometer, Mabis Healthcare Inc, 15-633-000, 50EA/BX 0.00 CALAMINE LOT, MAJOR PHARMACEUTICALS, INC., 115923,48EA/CS 0.00 Renaissance Ii Spiro Kit , Mallinckrodt, P-000725-00, Ea 420 0.00 421 Thermometer, Digital, Fahrenheit, Beeper, Mark Of Fitness, MF-1, 12EA/BX, 12BX/CS 0.00 422 Glove Exam, Non-Latex, PVC, Powdered, Large, Maxxim Medical, 484003, 100EA/BX, 10BX/CS 0.00 Mark Of Fitness Maxxim Medical Maxxim Medical MF-1 484003 6901-CVP019 423 Maxxim Medical MC010A 424 MEDEGEN MEDICAL PRODUCTS 4093 425 Tray, Surgical, Custom, Open Heart, Maxxim Medical, 6901CVP019, 1EA/CS 0.00 Stand Cover, Mayo, Reinforced, Maxxim Medical, MC010A, 50EA/CS 0.00 Irrigation Tray With 60Cc ,Graduated, Medegen Medical Products, 4093 0.00 40 426 Basin, Wash, Round, Blue, Autoclave, 3-7/8" X 12-3/8"DIA X 3-3/8", Medegen Medical Products - Vollrath, 40, 12EA/CS 0.00 63 427 Basin, Emesis, 9", Autoclave, 500CC, Powder Clue, Medegen Medical Products - Vollrath, 63, 24EA/CS 0.00 H362-05 428 Basin, Wash, Rectangular, 71/2QT, 6.6L, Medegen Medical Products - Vollrath, H362-05, 50EA/CS 0.00 Medegen Medical Products - Vollrath Medegen Medical Products - Vollrath Medegen Medical Products - Vollrath Medexco Supply Co MMM2206 Stethoscope, Classic S.E. Carib Blue, 28", Littman, Medexco Supply Co, MMM2206, EA NON02850 BAG WATER SOLUBLE 36X39 25", MEDICAL ACTION INDUSTRIES, NON02850,25EA/BX, 4BX/CS 429 MEDICAL ACTION INDUSTRIES 430 Medical Action Industries 50-20 431 Medical Action Industries 50-21 432 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 Patient Bag Set w/draw string, Medical Action Industries, 5020, 250/CS 0.00 Personal belongings bag, 20x22", White, Medical Action, 5021, 250/CS 0.00 Page 76 Medical Action Industries 2410 433 Medical Action Industries 8704 434 Medical Action Industries 8705 435 Medique Products 734M1 436 Biohazard Bag, 24"x23", Red , Medical Action Industries, 2410, 1000/CS 0.00 Sharps Collection Container, 2Gallon, Red, Medical Action Industries, 8704, 24/CS 0.00 Sharps Collection Container, 8 Gallon, Red, Medical Action Industries, 8705, 10/CS 0.00 Cabinet, Filled, 4 Shelf, MediFirst Tablets, Medique Products, 734M1, EA 0.00 73501B/G Travel Kit/Medique, Custom Ds, Medique, 73501B/G, Ea DYND-30335 438 Cup, Specimen, Polypropylene, 4OZ, Non-Sterile, Green on Lid, Medline Industries, DYND30335, 500EA/CS 0.00 NON27236 439 Gown, Isolation, Elastic Cuff, Spunbound, Large, Yellow, Medline Industries, NON27236, 50EA/CS 0.00 MEDIQUE PRODUCTS 437 0.00 Medline Industries Medline Industries MEDLINE INDUSTRIES 80301 440 MEDOVATIONS INC 1217-10 BASIN RECTANGL WASH, MEDLINE INDUSTRIES, 80301,50EA/CS 0.00 Nasogastric Tube 10Fr, Medovations Inc, 1217-10, Ea 441 0.00 Metrex 10-1624 442 Metrex 4397-MX-1510 443 Gel, Antiseptic, Hand wash, No Rinse, 4OZ, Flip Top, Metrex, 10-1624, 24EA/CS 0.00 Wipe, Skin, Antimicrobial, 5" X 7", Metrex, 4397-MX-1510, 50EA/BX, 10BX/CS 0.00 MPF105L/48216 LRG 444 LATEX EXAM GLV PWDR FREE LRG, MICROFLEX, MPF105-L/48216 LRG,100EA/BX, 10BX/CS 0.00 MPF105M/48215 MED 445 LATEX EXAM GLV PWDR FREE MED, MICROFLEX, MPF105-M/48215 MED,100EA/BX, 10BX/CS 0.00 MICROFLEX MICROFLEX MICROFLEX MP100-M/35114 MED 446 MICROFLEX MP100-L/35115 LG 447 MICROFLEX MP100-S/35201 SM 448 RFP No. 10-475-000-J Medical and Dental Supplies LATEX EXAM GLOVES MED, MICROFLEX, MP100-M/35114 MED,100EA/BX, 10BX/CS 0.00 LATEX EXAM GLOVES LRG, MICROFLEX, MP100-L/35115 LG,100EA/BX, 10BX/CS 0.00 LATEX EXAM GLOVES SML, MICROFLEX, MP100-S/35201 SM,100EA/BX, 10BX/CS 0.00 Page 77 MICROFLEX MPF105S/48214 SM 449 MICROFLEX MF300 LRG LATEX EXAM GLV PWDR FREE SML, MICROFLEX, MPF105-S/48214 SM,100EA/BX, 10BX/CS 0.00 Gloves, Diamond Grip Pf Lg, Microflex, Mf300 Lg, 100/Bx 450 0.00 MICROFLEX MF300 MED Gloves, Diamond Grip Pf Med, Microflex, Mf300 Med 100/Bx 451 0.00 MICROFLEX MF300 XLG Gloves, Diamond Grip Pf Med, Microflex, Mf300 Xlg, 100/Bx 452 0.00 MICROFLEX DGP-350-L Gloves, Diamond Grip Plus Pf Lg, Microflex, Dgp-350-L, Bx/100 453 0.00 MICROFLEX DGP-350-M 454 MICROFLEX DGP-350-XL 455 MILTEX INSTRUMENT 9-40-22 Gloves, Diamond Grip Plus Pf Med, Microflex, Dgp-350-M, Bx/100 0.00 Gloves, Diamond Grip Plus Pf Xlg, Microflex, Dgp-350-Xl, Bx/100 0.00 Orthodontic Wire, 22G, 1 Oz, Miltex Instrument, 9-40-22, Ea 456 0.00 MILTEX INSTRUMENT 4-111 Surgical Blade,#11, Miltex Instruments, 4-111,100/Bx 457 0.00 MILTEX INSTRUMENT 4-115 Surgical Blade,#15,Miltex Instruments,4-115,100/Bx 458 0.00 Miltex Instrument Co 33-36 459 Miltex Instrument Co 426-26330 460 Miltex Instrument Co 9D-135 461 Miltex Instrument Co BL120 462 Miltex Instrument Co BL130 Punch, Biopsy, Disposable, 6MM, Miltex Instrument Co, 3336, 50EA/BX 0.00 Separating Disk I, 7/8, Flat Double, Miltex Instrument Co, 426-26330, 100EA/BX 0.00 Scissor, Wire Cutting, 4-1/4" (10.8CM), Curved, Miltex Instrument Co, 9D-135, 1EA/BX 0.00 Forceps, Dressing, Serrated, 5", Miltex Instrument Co, BL120, 12EA/BX 0.00 Forceps, Splinter, 3.5", Miltex Instrument Co, BL130, 12EA/BX 463 0.00 Miltex Instrument Co BL132 Forceps, Splinter, 4.5", Miltex Instrument Co, BL132, 12EA/BX 464 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 78 Miltex Instrument Co BL200 465 Miltex Instrument Co V95-504SS 466 Forceps, Halstead, 5", Straight, Miltex Instrument Co, BL200, 12EA/BX 0.00 Scissor, Lister, 5.5", Miltex Instrument Co, V95-504SS, 1EA/BX 0.00 265324 467 Dressing, Composite, Adhesive Film, 4" X 4" All Dress, Molnlycke Health Care, 265324, 10EA/BX, 10BX/CS 0.00 265346 468 Dressing, Composite, Adhesive Film, 6" X 6", Molnlycke Health Care, 265346, 10EA/BX, 10BX/CS 0.00 Molnlycke Health Care Molnlycke Health Care Nellcor/Puritan Bennett 6DIC 469 Inner Cannula, Disposable, Size 6, Nellcor/Puritan Bennett, 6DIC, 10EA/BX 0.00 B3158-1 Saline Blood Bank, 1 X 20 Liter, NERL Diagnostics Inc, B3158-1, EA 9871616091 471 Diet, Elemental, Isotonic, Liquid, Pep amen, 1500ML, Nestle Clinical Nutrition, 9871616091, 1500ML/BG, 4BG/CS 0.00 10701570 472 Bandage, Adhesive, Flexible, Latex Free, X-Large, 2" X 4-1/2", NutraMax, 10701570, 50EA/BX, 24BX/CS 0.00 NERL Diagnostics Inc 470 Nestle Clinical Nutrition NutraMax NutraMax 9111-000 0.00 First Aid Kit, Plastic, Basic, NutraMax, 9111-000, 12EA/CS 473 0.00 OPTI-KLENS OPTIKLENS Eye Wash, Opti-Klens, OptiKlens, Ea 474 0.00 PACIFIC MANAGEMENT CRV-13 475 PACIFIC MANAGEMENT CRV-16 476 PACIFIC MANAGEMENT CRV-20 477 PACIFIC MANAGEMENT CRV-30 478 PACIFIC MANAGEMENT CRV-40 479 PACIFIC MANAGEMENT CRV-60 480 RFP No. 10-475-000-J Medical and Dental Supplies Vials, Rx, 13Dr, Child Proof, Pacific Management, Crv-13, 250/Cs 0.00 Vials, Rx, 16Dr, Child Proof, Pacific Management, Crv-16, 200/Cs 0.00 Vials, Rx,20Dr, Child Proof, Pacific Management, Crv-20, 150/Cs 0.00 Vials, Rx, 30Dr, Child Proof, Pacific Management, Crv-30, 120/Cs 0.00 Vials, Rx, 40Dr, Child Proof, Pacific Management, Crv-40, 250/Cs 0.00 Vials, Rx, 60Dr, Child Proof, Pacific Management, Crv-60, 60/Cs 0.00 Page 79 Vials, Rx, 8Dr, Child Proof, Pacific Management, Crv-08, 380/Cs PACIFIC MANAGEMENT 481 PALCO PCL300 0.00 Pulse Oximeter, Sensor, Palco Laboratories, Pcl300, Ea /Bx 482 0.00 Palco Laboratories 010-300 Oximeter, Hand-Held, Palco Laboratories, 010-300, EA 483 0.00 Palmero Health Care 60DIS 484 PARKER LABS 4001-32GF 485 PECHINEY PLASTIC PACKAGING PM992 Towelette, Disinfectant, ORM, Palmero Health Care, 60DIS, 60EA/BX, 12BX/CS 0.00 ULTRASOUND GEL 1-LIT, PARKER LABS, 400132GF,6EA/BX, 2BX/CS 0.00 Parafilm, 2"X250', Pechiney Plastic Packaging, Pm992, Bx 486 0.00 155ND-41 Scale, Professional Dial, Pelstar, 155Nd-41, Ea 70-150 488 Mask, Microshield CPR Original, Orange Pouch, Medical Devices International, 70-150, 10EA/BX, 5BX/CS 0.00 30500 489 Cup, Alpha, Clear, Disposable, Polypropylene, 5OZ, Polar Plastics, 30500, 50EA/PK, 50PK/CS 0.00 490 SHARPS CONTAINER 1.5GL2201LPBW, POST MEDICAL, INC., 2201LPBW,22EA/CS 0.00 PELSTAR 487 0.00 Medical Devices International Polar Plastics POST MEDICAL, INC. PRECISION DYNAMICS CORP 2201LPBW 45006-15-MPN 491 Premier 9308W Arm board, Infant, 2X6" Reusable, Precision Dynamics Corp, 45006-15-Mpn 0.00 Slides, adhesive, 25mm x 75mm, Premier, 9308W, 72/BX 492 0.00 493 Slipper, Original, Foam, X-Large, Non-Skid Soles, Latex Free, Principle Business Enterprises, 5007, 72PR/CS 0.00 Q35372 494 Wipe, Hand Cleaner, Antimicrobial, Regular, 6" X 71/2", Professional Disposables, Q35372, 135EA/BX, 12BX/CS 0.00 Q08472 495 SANI-CLOTH HB TUB/160, PROFESSIONAL DISPOSABLES, Q08472,160EA/BX,12BX/CS 0.00 Principle Business Enterprises Professional Disposables PROFESSIONAL DISPOSABLES PROFESSIONAL HEALTH 5007 Q89072 496 RFP No. 10-475-000-J Medical and Dental Supplies Sani Cloth Plus Germ Wipe Large, Professional Disp, Q89072, Ct 0.00 Page 80 PROFFESSIONAL DISPOSABLES J15095 497 PROMAR INDUSTRIES, INC. 5816MRE 498 Propper 265102 Wipe, Personal Cleansing Washcloth, Professional Disposables, J15095 0.00 PEN LIGHT-DIAGNOSTICDISP, PROMAR INDUSTRIES, INC., 5816MRE,6EA/BX, 60BX/CS 0.00 Indicator Strip, Steam, Small Strip, Propper, 265102, 250/BX 499 0.00 PROPPER 24015 Autoclave Pouch, 5X15, Propper, 24015, 250/Bx 500 0.00 PROPPER 24014 Autoclave Pouch, 8X16, Propper, 24014.125/Bx 501 0.00 PURDUE FREDERICK CO 2100-87 502 BETADINE SOL, PURDUE FREDERICK CO, 210087,12EA/CS 504 APPLICATOR COTTON TIP 6WOOD, PURITAN MEDICAL PROD CO LLC, 258062WCAL,100EA/BX, 10BX/CS BLADE TONGUE 6IN STERILE SENIOR, PURITAN MEDICAL PROD CO LLC, 25-705ALL,100EA/BX, 10BX/CS 505 BLADE TONGUE 6 SENIOR N/S, PURITAN MEDICAL PROD CO LLC, 704ALL,500EA/BX, 10BX/CS PURITAN MEDICAL PROD CO LLC 258062WCAL 503 PURITAN MEDICAL PROD CO LLC PURITAN MEDICAL PROD CO LLC QUALITY SCIENTIFIC PLASTICS 25-705-ALL 704ALL A20041ALG 506 Regent Medical 0575-16 507 0.00 0.00 0.00 0.00 Tube, Culture, 12X75Mm, Ps, Quality Scientific Plastics, A20041Alg, 1000/Cs 0.00 Cleanser, Skin, Hibiclens, 16OZ, Chlorhexidine, Regent Medical, 0575-16, 12EA/CS 0.00 10131 508 Syringe, 1CC, 27G X 1/2", Tuberculin, Vanish point, Retractable Technologies, Inc., 10131, 100EA/BX, 8BX/CS 0.00 10211 509 Syringe, 1CC, 29G X 1/2", Insulin, Vanish point, Retractable Technologies, Inc., 10211, 100EA/BX, 8BX/CS 0.00 Retractable Technologies, Inc. Retractable Technologies, Inc. Roche 553 Test Strips, Comfort Curve, Roche, 373, 50/BX 510 0.00 Roche 200743 511 RFP No. 10-475-000-J Medical and Dental Supplies Test Strips, ChemStrip 2GP Urinalysis, Roche, 200743, 100/BX 0.00 Page 81 Roche 417109 512 Roche 417145 513 Rondex Products, Inc 3030-MOORE 514 Ross Product Division 50460 515 Safetec Of America, Inc. 17100 516 Test Strips, ChemStrip 9 Urinalysis, Roche, 417109, 100/BX 0.00 Test Strips, ChemStrip 10 Urinalysis, Roche, 417145, 100/BOX 0.00 Valve, CPR, Safety, Latex Free, Standard, Rondex Products, Inc, 3030-MOORE, EA 0.00 Supplement, Ensure, Diet, Vanilla, Ross Product Division, 50460, 24EA/CS 0.00 Kit, Universal, Precaution, Polybag Container, Safetec Of America, Inc., 17100, 24EA/CS 0.00 17350 Cleaner, Hand Sanitizer, FlipTop Bottle, 4OZ, Safetec Of America, Inc., 17350, 24EA/CS 34105 518 Disinfectant, Fungicidal, Antibacterial Deodorant, Safetec Of America, Inc., 34105, 16OZ/EA, 12EA/CS 0.00 34400 519 Wipe, Antimicrobial, w/ Aloe Vera & PCMX, Safetec Of America, Inc., 34400, 100EA/BX, 10BX/CS 0.00 520 Glove, Safety, Latex, X-Large, 15ML at Fingertips, Sage Products Inc., BS0480-1, 50EA/BX, 20BX/CS 0.00 Safetec Of America, Inc. 517 Safetec Of America, Inc. Safetec Of America, Inc. Sage Products Inc. SHAMROCK MARKETING CO, INC BS0480-1 16013 521 SHAMROCK MARKETING CO, INC 16012 522 SHAMROCK MARKETING CO, INC 16011 523 SHAMROCK MARKETING CO, INC 16014 524 SHAMROCK MARKETING CO, INC 11113 0.00 Gloves, Action, Latex, Pf, Lg, Shamrock Marketing Co., Inc, 16013, Cs/100 0.00 Glove, Action, Latex, Pf, Med, Shamrock Marketing Co, Inc, 16012, Cs/100 0.00 Glove, Action, Latex, Pf, Small, Shamrock Marketing Co, Inc, 16011, Cs/100 0.00 Glove, Action, Latex, Pf, Xlg, Shamrock Marketing Co, Inc, 16014, Cs/100 0.00 Gloves, Econ, Latex Pwd Lg, Shamrock, Bx 525 0.00 SHAMROCK MARKETING CO, INC 11112 Gloves, Econ, Latex Pwd Med, Shamrock, Bx 526 0.00 SHAMROCK MARKETING CO, INC 11111 Gloves, Econ, Latex Pwd Small, Shamrock, Bx 527 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 82 SHAMROCK MARKETING CO, INC 11114 Gloves, Econ, Latex Pwd Xlg, Shamrock, Bx 207026 529 Cup, Specimen, Packaged Individually, Sterile, Green Cap, 4OZ, Sherwood, 207026, 100EA/CS 0.00 207034 530 Cup, Specimen, Bulk, NonSterile, White Cap, 4OZ, Sherwood, 207034, 50EA/BX, 10BX/CS 0.00 528 0.00 Sherwood Sherwood Sherwood 221000 Probe Cover, First FILAC F1500, Sherwood, 221000, 20EA/PK, 25PK/BX, 10BX/CS 810055 Probe Cover, Disposable, Single Use, w/ First Temp & Genius Thermometer, Sherwood, 810055, 100EA/BX, 20BX/CS 531 Sherwood 532 Skil-Care Corp 102010 0.00 0.00 Bag, Holder, Urinary, Drainage, Skil-Care Corp, 102010, 1EA/BX 533 0.00 SMITH & NEPHEW 4210 Dressing, Hypafix Sheet, 4 X 10 Yd, Smith & Nephew, 4210, Bx 534 0.00 SMITH & NEPHEW 5941-402300 535 SOLON 320-00 536 SOLON 362 Unisolve Adhesive Remover Pad, Smith & Nephew, 5941402300, Bx 0.00 TONGUE DEPRESSOR SEN, SOLON, 320-00,500EA/BX, 10BX/CS 0.00 Applicator, 6", N/S, Solon, 362, 1000/Bx 537 0.00 SOLON 368 Applicator, 6", Sterile, 2'S, Solon, 368, 100 2'S/Bx 538 0.00 SOLON 376 Applicator, 8", Ob/Gyn, Solon, 376, 500/Cs 539 0.00 SOLON 35900 Applicator Dowels, Wooden, 6", Solon, 35900, 1000/Bx 540 0.00 SOLON 36215 Applicators, Cotton Tip, 6", N/S, Solon, 36215, 100/Bx 541 0.00 SOLON 300-00 Tongue Depressor, Jr, Solon, 500/Bx 542 0.00 SOLON 369 Tongue Depressor, Sr, Solon, 369, 100/Bx 543 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 83 STAYFREE 146-7125 544 STRYKER MEDICAL 6092 STAYFREE MAXI PAD BG, STAYFREE, 146-7125,24EA/BX, 12BX/CS 0.00 Stryker Ez Pro, R4, Stryker Medical, 6092, Ea 545 0.00 STRYKER MEDICAL 6082 Stryker 6082, Ea 546 0.00 STUART PHARM 0575-91 Soap, Hibiclens, Liquid, Stuart Pharm, 0575-91, Bx 547 0.00 Sultan Chemists 72010 Kit, Versa Temp, 4 Shades, Gun, Sultan Chemists, 72010, EA 548 0.00 Sultan Chemists 95029 549 Sunrise Medical HHG, Inc 4650D-621 550 Sunrise Medical HHG, Inc 5650D 551 SUURETEMP 01679-400 Lysol, Disinfectant, IC, 19OZ, Acc-U-Sol, Sultan Chemists, 95029, 12EA/CS 0.00 Kit, Accessory for DeVilbiss Pulmo-Aide, Sunrise Medical HHG, Inc, 4650D-621, 50EA/CS 0.00 Nebulizer, Compressor, Aerosol, 10ML Nebulizer Filter, Sunrise Medical HHG, Inc, 5650D, EA 0.00 Thermometer, Sure Temp Electronic, 01679-400, Ea 552 0.00 Sweet Paper 54039 553 TEC LABORATORIES INC CALA-6 Cup, Paper, Wax, 3OZ, Sweet Paper, 54039, 100EA/BG, 50BG/CS 0.00 CalaGel Medicated, Tec Laboratories Inc, Cala-6, Oz 554 0.00 Tech Med Services Inc. 4415 555 TECH MED SERVICES INC. 3050 556 Envelope, Pill, Imprinted, 3" X 2", Tech Med Services Inc., 4415, 1000EA/BX 0.00 TRAUMA DRESSING 3050 HP, TECH MED SERVICES INC., 3050,25EA/CS 0.00 DYKD1STAID25 First Aid Kit, 25 People, Technicality, Inc, Dykd1Staid25, Ea 6293-NN2038R 558 Needle, 20G X 1-1/2", UltraThink Wall, Terumo Corporation, 6293-NN2038R, 100EA/BX, 10BX/CS 0.00 SROX2032CA 559 IV Catheter, Teflon, SurFlo, 20G X 1-1/4", Terumo Corporation, SROX2032CA, 50EA/BX, 4BX/CS 0.00 TECHNICALITY INC 557 Terumo Corporation Terumo Corporation RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 84 TERUMO CORPORATION MN2000T Multi Sampler Adapter, Terumo Corporation, Mn2000T, Bx 560 0.00 TERUMO CORPORATION MN*SVS23B30 Syringe,Surshield,23Gx3/4 12",Tb,Terumo,Mn*Svs23B30,Bx 561 0.00 TERUMO CORPORATION SS10M2913 Syringe, Insulin, 29G, 1Cc, Terumo, Ss10M2913, Bx 562 0.00 TILLOTSON 4910/03-2542 Bandage, Triangle, Tillotson , 4910/03-2542, Ea 563 0.00 TRIAD DISPOSABLES 1169416 Antiseptic Towelettes, Triad Disposables, Inc, 1169416, Bx 564 0.00 TRIAD DISPOSABLES 402772 Alcohol Prep Pad, Large, Triad, 402772, Bx 10-5201 Towelette, Antiseptic, Disposable, No Alcohol, Triad Disposables, Inc., 10-5201, 100EA/BX, 10BX/CS 565 0.00 Triad Disposables, Inc. 566 Triad Disposables, Inc. 10-3001B Pad, Isopropyl, Sterile, Medium, Triad Disposables, Inc., 103001B, 200EA/BX, 10BX/CS 402407 ALCOHOL PREP PDS MD 103001ORM, TRIAD DISPOSABLES, INC., 402407,200EA/BX, 20BX/CS 567 TRIAD DISPOSABLES, INC. 568 Troy 220099 569 U.S. Cotton, LLC 79210500 570 UNISOURCE KC03076 0.00 0.00 0.00 Swab, Culture, Liquid, Stuart Single, Troy, 220099, 50EA/BX, 10BX/CS 0.00 Cotton Ball, Medium, Sterile, U.S. Cotton, LLC, 79210500, 500EA/BX, 12BX/CS 0.00 Kleenex Facial Tissue White, Unisource, Kc03076, Cs 571 0.00 Unisource - WindsorSupplier-Inv KC21340-80 572 United Metal Fabricators, Inc. 5140 573 United Metal Fabricators, Inc. 6117 574 United Metal Fabricators, Inc. 6204 575 RFP No. 10-475-000-J Medical and Dental Supplies Tissue, Facial, 2Ply, Surpass, Unisource - Windsor- SupplierInventory, KC21340-80, 100EA/BX, 30BX/CS 0.00 Table, Exam, w/ Pelvic Tilt, One Touch Stirrups, United Metal Fabricators, Inc., 5140, EA 0.00 Cabinet, Treatment, w/ Lock, 32"W X 65"H X 16-1/4"D, United Metal Fabricators, Inc., 6117, EA 0.00 Cabinet, Mobile Treatment, w/ Four Drawers, United Metal Fabricators, Inc., 6204, EA 0.00 Page 85 United Metal Fabricators, Inc. 6620 576 United Metal Fabricators, Inc. 8425 577 Cart, EKG, w/ 5 Turn Wheel, Casters, 2 Drawers, United Metal Fabricators, Inc., 6620, EA 0.00 Triple Panel Screen, Individual Casters, United Metal Fabricators, Inc., 8425, EA 0.00 578 Blanket, Bath, Economy, Unbleached, 70" X 90", Universal Weavers Corporation, MDT218242, 24EA/CS 0.00 579 Jacket, Extra Safe, Hip Light, Pink, Large, Valumax International, 3630LPL, 10EA/BG 0.00 Universal Weavers Corporation Valumax International Viro Research MDT218242 3630LPL MX-1518 580 Vital care 24-115200 581 VOLLGRATH GROUP 5216-2602 Soap, Liquid, Antimicrobial, 18OZ, Bottle w/ Pump, Viro Research, MX-1518, 12EA/CS 0.00 Specula, Vaginal, Plastic, Clear, Small, 24-115200, 10EA/BG, 10BG/CS 0.00 Iv Start Kit With Tegaderm, Vollrath Group, 5216-2602, Ea 582 0.00 VOLLGRATH GROUP H21501 Pitcher Liner, 1Qt, Clear, Vollrath Group, H21501, Cs 583 0.00 Wampole Laboratories 84136 584 Test Mono-Plus II, Cassette, Wampole Laboratories, 84136, 30EA/BX 0.00 135007 Test, Strip, Strep A, Clearview, Cassette, Wampole Laboratories, 135007, 30EA/BX 05031-101 586 Probe Cover, Disposable, For Sure Temp Thermometer, Welch Allyn, 05031-101, 25EA/PK, 10PK/BX, 4BX/CS 0.00 587 Monitor, Vital Signs, Blood Pressure, Temperature, Mobile Stand, Welch Allyn, 42NTB-E1M, EA 0.00 Wampole Laboratories 585 Welch Allyn Welch Allyn Welch Allyn 42NTB-E1-M 05074-800 588 WELCH ALLYN 72200 0.00 Probe Covers for Pro-1 Thermometer, Welch Allyn, 074800, 200/BX 0.00 Battery, 3.5V Rechargeable, Welch Allyn, 72200, Ea 589 0.00 WELCH ALLYN 72300 590 WELCH ALLYN 7800 Battery, 3.5V Rechargeable, Audiscope, Welch Allyn, 72300, Ea 0.00 Bulb, Halogen Lamp 6, Welch Allyn, 7800, Ea 591 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 86 WELCH ALLYN 6783-44500 Exam Light, Welch Allyn, 678344500, Ea/Bx 592 0.00 WELCH ALLYN 76793 Integrated Sys W/767 Trans , Welch Allyn, 76793, Ea 593 0.00 WELCH ALLYN 78810 Kleenspec Sys, Welch Allyn, 78810, Ea 594 0.00 WELCH ALLYN 11710 Ophthalmoscope Head, Welch Allyn, 11710, Ea 595 0.00 WELCH ALLYN 03100-U Otoscope Bulb, Welch Allyn, 03100-U, Ea 596 0.00 WELCH ALLYN PC800 Thermometer Probe Covers, Welch Allyn, Pc800, Cs 597 0.00 WELCH ALLYN 58000 Specula, Kleenspec, Small, Welch Allyn, 58000, Bx 598 0.00 WELCH ALLYN 52432 599 WELCH ALLYN 52434 Specula, Otoscope, 2.5,Disp,Welch Allyn, 52432, 10000/Cs 0.00 Specula, Otoscope, 4Mm, Disp, Welch Allyn, 52434, 1000/Ct 600 0.00 WELCH ALLYN 58004 Speculum, Kleenspec, Lg, Welch Allyn, 58004, 20/Bx 601 0.00 WELCH ALLYN 58001 Speculum, Kleenspec, Med, Welch Allyn, 58001, 20/Bx 602 0.00 WELCH ALLYN 01679-200 Thermometer, Oral Probe, Welch Allyn, 01679-200, Ea 603 0.00 WELCH ALLYN 76710 Transformer Wall 3.2V W/O Clock, Welch Allyn, 76710, Ea 604 0.00 Vital Sign Monitor, Welch Allyn, 4200B-E1, Ea WELCH ALLYN 605 0.00 Winfield 8702 606 RFP No. 10-475-000-J Medical and Dental Supplies Collector, Sharps, Red, 1 QT, 3.5"W X 3.5"D X 7"H, Winfield, 8702, 72EA/CS 0.00 Page 87 Dental Supplies Manufacturer 3M ESPE Mfg SKU 7542 Description 116K Biological Monitor System, Attest, 116K, 1 complete system 3411 Composite Adhesive, 3m Adper Single bond Plus Refill, 3411, 6 Gram Bottle 2 3M ESPE 3 3M ESPE 1262P List price Discount off List Price to State $0.00 30% 0.00 $0.00 30% 0.00 0.00 Biological Monitor System, Attest Refill , 1262P, 25/Box 4 0.00 3M ESPE PDRP-ELR2 Crowns, Nichro ELR2, PDRP-ELR2, 5/Box 5 0.00 3M ESPE PDRP-ELR4 Crowns, Nichro ELR4, PDRP-ELR4, 5/Box 6 0.00 3M ESPE 1942FR 7 3M-CF 3M-CF PDRP-ELL3 ND96 Crown & Bridge Products, Crowns Nichro Starter, 3M-Cf, Nd96, 96/Bx DS-8011-00 GLOVES, LATEX P/F TEXT N/S, SMALL, GLOVETEX, AHP, DS-8011-00, 100/BX DS-8012-00 GLOVES, LATEX P/F TEXT N/S, MEDIUM, GLOVETEX, AHP, DS-8012-00, 100/BX DS-8013-00 GLOVES, LATEX P/F TEXT N/S, LARGE, GLOVETEX, AHP, DS-8013-00, 100/BX 90800 X-RAY CHEMICALS, DEVELOPER & FIXER, QUARTS, PERI-PRO, AIR TECHNIQUES, 90800, 6/CS 9 AHP 10 AHP 11 AHP 12 AIR TECHNIQUES MASKS, FLUID RESISTANT, ROSE, MOLDED, 3M ESPE, 1942FR, 50/BX Crown & Bridge Products, 3M Crowns Ss 2Nd Prim Mol, E-Ll3, 3M-Cf, Pdrp-Ell3, 5/Bx 8 13 Distributer SKU Composite Primer, Adper Scotchbond MP, 7542, 8ml 1 3M ESPE UoM RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 88 AIR TECHNIQUES 40270 14 AIR TECHNIQUES 43945 15 Alpha Protec 608 16 ANSELL 5761 X-RAY CHEMICALS, DEVELOPER & FIXER, GALLONS, AIR TECHNIQUES, 90270, 4/CS X-RAY PROCESSOR CLEANER, FORMULA 2000, FOR TANK & TRANSPORT ROLLERS, HAZMAT FEE EXTRA, AIR TECHNIQUES, 43945, 2BTL/PK Face Mask, Magic Arch w/ Retention Band, Alpha Protec, 608, 50/Box 0.00 0.00 Gloves, Micro-Touch Latex Glove, Large, Ansell, 5761, 100/Bx 17 0.00 ANSELL 5741 Gloves, Micro-Touch Latex Glove, Medium, Ansell, 5741, 100/Bx 18 0.00 ANSELL 5721 Gloves, Micro-Touch Latex Glove, Small, Ansell, 5721, 100/Bx 5006 GLOVES, LATEX P/F N/S, LARGE, MICROTOUCH PLUS, ANSELL PERRY, 5006, 100/BX 19 0.00 ANSELL PERRY 20 Benco Dental 2530-352 Mix Tips, High Performance Teal, Benco Dental, 48/Box 2403-481 Hand piece High Speed, Hy-Torq 4-hole with Bur Tool, Benco Dental 3247-747 Toothbrush Adult Iris Straight, Benco Dental, 3247-747, 72/CS 21 0.00 0.00 Benco Dental 22 Benco Dental 23 Benco Dental 3247-765 0.00 0.00 Toothbrush Adult, Iris Curved, Benco Dental, 3247-765, 72/CS 24 0.00 Benco Dental 207MLP-2N Lab Work Pan. Neon Blue, Benco Dental, Each 6005PFL GLOVES, NITRILE P/F N/S, LARGE,NDEX, BEST MANUFACTURING, 6005PFL, 100/BX 25 0.00 BEST MANUFACTURING 26 Buffalo Mfg. 02701S Arbor Bands, 3/4 in. Course, Buffalo, 02701S, 100/Box 872P Preventive Products, Go Betweens Cleaners, Butler, 872P, 36Pk/Bx 27 0.00 0.00 BUTLER 28 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 89 CAULK 610007-6 Cements - Liners Bases, Ivory Complete Package, Caulk, 610007-6, Ea 624055-6 Finishing & Polishing, Enhance Finishing Refill, Cups, Caulk, 624055-6, 40/Bx 29 CAULK 30 CAULK 608522 Impression Materials & Access, Jeltrate Fast Set, 1Lb, Caulk, 608522, Each 605602 Impression Materials & Access, Jeltrate Plus Fast Set, Caulk, 605602, 1Lb 31 CAULK 32 CAULK 643300 33 CAULK 685611 Restorative Materials, Dyract Flow Syringe Refill, A2, Caulk, 685611, Ea 634352-6 Restorative Materials, Prime & Bond Refill Sq, 4.5 Ml, Caulk, 634352-6, 2/Bx 656-2862 Amalgam, Dispersalloy Capsule Fast Set, 600mg (2 spill), Caulk 656-2862, 50/Box 34 CAULK 35 Caulk 36 CLIVE 31526 Dental Hand Instruments, Tub Lid Clear Standard, Size S, Clive, 31526, Ea 31970 Dental Hand Instruments, Tub Standard Size S, Lilac, Clive, 31970, Ea 37 CLIVE 38 CLIVE CRAIG 31626 39 COTTRELL DT-OOW 40 COTTRELL PNS100 41 CRESNT Preventive Products, Fluroshield Standard Pack, Stand, Caulk, 643300, Ea STERILIZATION POUCHES, PEELVUE, 3 1/4"X12", SELF SEAL, CLIVE CRAIG, 31626, 200/BX SETUP TRAYS, PROTRAY, DISPOSABLE WHITE, DIVIDED, COTTRELL, DT-OOW, 200/CS NEEDLE SHEATH, PROP, 2 1/2"X3 1/4", PROTECTOR, COTTRELL, PNS100, 100/BX C02-0200 Dental Small Equipment, Wig-L-Bug Model, C02-0200, Ea GCSSS FACE SHIELD, FOAM HEADBAND, 13" W X 7.5" L, DISPOSABLE, CROSSTEX, GCSSS, 24/BX 42 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 CROSSTEX 43 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 90 CROSSTEX GCIBL MASKS, ISOFLUID, BLUE, EARLOOP, CROSSTEX, GCIBL, 50/BX GCIPK MASKS, ISOFLUID, PINK, EARLOOP, CROSSTEX, GCIPK, 50/BX 44 CROSSTEX 45 DASH LP100M 46 DASH LP100S 47 DASH NV100S GLOVES, VINYL P/F N/S, SMALL, NUVO, DASH, NV100S, 100/BX TPF100M GLOVES, LATEX P/F TEXT N/S, MEDIUM, MICROPRO, TPF100M, 100/BX TPF100XS GLOVES, LATEX P/F TEXT N/S, X-SMALL, MICROPRO, TPF100XS, 100/BX 48 DASH 49 DASH 50 DENTAL DISPOSABLES INTL SNG-150 51 DENTAL DISPOSABLES INTL SS-500 52 DENTAL DISPOSABLES INTL TLC-500 53 Dentamerica GLOVES, LATEX POWDER N/S, SMALL, LP EXAM, DASH, LP100S, 100/BX GLOVES, LATEX POWDER N/S, MEDIUM, LP EXAM, DASH, LP100M, 100/BX CHAIR COVER, SLIPN-GRIP, 48"X56", DENTAL DISPOSABLES, SNG150, 150/BX SYRINGE SLEEVE, WITH OPENING, 2.5"X10", DENTAL DISPOSABLES, SS500, 500/BX LIGHT SLEEVE, TSTYLE, CLEAR PLASTIC, DENTAL DISPOSABLES, TLC500, 500/BX 680A-110 Curing Light, 680 Halogen, Dentamerica 680A-110, Each 81302 Cavitron Select SPS Ultrasonic Scaler Unit, 30k, Dentsply, 81302, 1 Unit 54 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Dentsply 55 Dentsply 130030 56 DENTSPLY PHARMACEUTICAL 54014 57 DENTSPLY RINN 58 0.00 21-6021 Chlorhexidine Gluconate Rinse, 0.12%, Dentsply, 130030, 16oz Bottle ANESTHETIC, CITANEST FORTE 4% W/ EPINEPHRINE, 1:200000, DENTSPLY PHARMACEUTICAL, 54014, 100/BX X-RAY MOUNTS, EZVIEW POCKET MASKED, 2H #2, BLACK, DENTSPLY RINN, 21-6021, 100/BX RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 Page 91 DENTSPLY RINN 54-0865 X-RAY POSITIONER, AIM RING, ANTERIOR BLUE, XCP/BAI, DENTSPLY RINN, 54-0865, EACH 54-0900 MOUTH PROPS, EZ PROP, DISPOSABLE, DENTSPLY RINN, 540900, 100/PK 54-0927 X-RAY POSITIONER, BITEWING ARM, RED, XCP/BAI, DENTSPLY RINN, 540927, EACH 59 DENTSPLY RINN 60 DENTSPLY RINN 61 Dentsply 54-0857 62 Dentsply 54-2001 63 DNTEQU 80395 6374106 Dental Small Equipment, Cavitron Insert 30K, 30K-10, 6374106, Ea 6374102 Dental Small Equipment, Cavitron Insert Tfi-3, 6374102, Ea 65 DNTEQU 66 DNTEQU XCP/BAI Kit, Evolution 2000 XCP Kit, Dentsply, 54-2001, 1 Each Dental Small Equipment, Cavitron 30K Sli-10S, Insert, 80395, Ea 64 DNTEQU XCP/BAI Color Coded Arm Anterior, Blue, Dentsply, 54-0857, 1 Each 26961 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Preventive Products, Delton Direct, 26965, 26961, Ea 67 0.00 DNTEQU 2896 Preventive Products, Delton Plus Refill, 28965, 2896, 50/Bx 31990 Dental Hand Instruments, Tub Insert Style S Clear, Dux, 31990, Ea (replaces #1356734) 68 0.00 DUX 69 Eastman-Kodak 198-7627 Panoramic Film, TMG RA5, 5x12,Eastman Kodak,1987627,50/Box 867-5332 PA Film, Insight IP-01 Poly, Size 0,Single Pack, Eastman Kodak, 867-5332, 100/Box 1163401 X Ray Film, Insight IP21 Poly Single #2, Eastman Kodak 1163401, 150/Box 122-8840 X Ray Film, Df-54 Poly Single #0, Eastman Kodak 1228840, 150/Box 70 Eastman-Kodak 71 Eastman-Kodak 72 Eastman-Kodak 73 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 Page 92 Eastman-Kodak 1273747 X Ray Film, Df-56 Poly Single #1, Eastman Kodak 1273747, 150/Box 1539931 X Ray Film, Insight IP21C Poly Single #2, Eastman Kodak 1539931, 100/Box 1658194 X Ray Film, Df-58 Poly Single #2, Eastman Kodak 1658194, 150/Box 1717131 X Ray Film, Df-58C Poly Single #2, Eastman Kodak 1717131, 100/Box 1878297 Anesthetic, Cartridges 0.5% w/ Epinephrine 1:200k, Eastman Kodak 1878297, 50/Can 0.00 X Ray Film Panoramic, XDBF-76 6x12, Eastman Kodak 1990712, 50/Box 0.00 74 Eastman-Kodak 75 Eastman-Kodak 76 Eastman-Kodak 77 Eastman-Kodak 78 Eastman-Kodak 1990712 79 Eastman-Kodak 1124981 X Ray Film, Insight IP11 Paper #1 Single, Eastman Kodak 1124981, 100/Box 1753664 X Ray Film, Df-57 Poly Double #2, Eastman Kodak 1753664, 150/Box 67-9L Dental Lab Equipment, Auto-Chuck Attach Arbor, Left, Handle, 67-9L, Ea 80 Eastman-Kodak 81 HANDLR 82 HU-FRIEDY EXS23 83 J&J 2683 STERILANTS, CIDEX PLUS 3.4% W/ACTIVATOR, J&J, 2683, 4QT/CS 2785 STERILANTS, CIDEX PLUS 3.4% W/ACTIVATOR, J&J, 2683, 1 GALLON 84 J&J 85 J&J INSTRUMENTS 07-898 86 J&JDNT 9864 87 J&JDNT 88 INSTRUMENT, EXPLORER #23, SINGLE END, HUFRIEDY, EXS23, EACH 9667 CROWN REMOVER, MORRELL W/ A-B-C TIPS, J&J INSTRUMENTS, 07898, KIT Preventive Products, Dental Floss Mint Wax 9864, 5Yd, 9864, 144/Bx (replaces #5552008) Preventive Products, Reach Floss Clean Burst, B-Mint, 9667, 144/Bx (replaces #5550782) RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 93 JOHN O. BUTLER CO 217P TOOTHBRUSH, GUM RAZZLE DAZZLE, JOHN O. BUTLER, 217P, 12/PK 612G Suture, Plain Gut , 3-0 X-1 18", Johnson and Johnson, 612G, 12/Box 89 Johnson and Johnson 90 KAVO 0524.5250 Hand Pieces, 14K Straight Attachment, 14K 4:1, Kavo, 0524.5250, Ea 0534.5330 Hand Pieces, Intra K Angle Attachment, 20K 1:1, Kavo, 0534.5330, Ea 28421 Impression Material, Extrude MPV (Dark Green), 50ml Cartridge, Kerr 28421, 32/Box 29946 Amalgam, Tytin Regular Set Capsule, 400mg (1 spill), Kerr 29946, 500/Jar 29949 Amalgam, Tytin Regular Set Capsule, 600mg (2 spill), Kerr 29949, 500/Jar 30768 Amalgam, Sybraloy Fast Set Capsule, 600mg (2 spill), Kerr 30768, 500/Jar 91 KAVO 92 Kerr 93 Kerr 94 Kerr 95 Kerr 96 Kerr 910070 Curing Light, Optilux 501 Halogen, Kerr 910070, Each 29948 Amalgam, Tytin Capsule Regular Set, 600 mg (2 spill), Kerr 29948, 50/Box 29967 Amalgam, Contour Capsule Regular Set, 600 mg (2 spill), Kerr 29967, 500/Jar 28747 Crown & Bridge Products, Kerr Extruder Gun 50Ml, Cartridge, Kerr, 28747, Ea 97 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Kerr 98 Kerr 99 KERR 100 KERR 27877 Impression Materials & Access, Extrude Putty, Kerr, 27877, Ea 20182 Restorative Materials, Herculite Enamel Refill, Univ, Kerr, 20182, 5Gm/Ea 29800 Restorative Materials, Herculite Enamel , A3.5, Kerr, 29800, 5Gm/Ea 29354 Restorative Materials, Prodigy Condensable Refill, B1, Kerr, 29354, 20/Bx 101 0.00 0.00 0.00 0.00 KERR 102 KERR 103 KERR 104 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 Page 94 KERR 25898 Restorative Materials, Prodigy Syringe, A1, Kerr, 25898, 4Gm/Ea 105 0.00 KERR 31211 Restorative Materials, Prodigy Syringe, A2, Kerr, 31211, 4Gm/Ea 106 0.00 KERR 31212 Restorative Materials, Prodigy Syringe, A3, Kerr, 31212, 4Gm/Ea 107 0.00 KERR 31213 Restorative Materials, Prodigy Syringe, A3.5, Kerr, 31213, 4Gm/Ea 108 0.00 KERRCS 00432 Impression Materials & Access, Impression Compound Stick, Red, Kerr, 00432, 15/Bx 148 MASKS, EARLOOP PROCEDURE, BLUE, FLUIDSHIELD, KIMBERLY CLARK, 000148, 40/BX 69980 COVER GOWN, CONTROL, X-LARGE, BLUE, KIMBERLY CLARK, 69980, 10/PK 109 KIMBERLY CLARK 110 KIMBERLY CLARK 111 KIMBERLY CLARK 47107 112 KIMBERLY CLARK 53431 113 KIMBERLY CLARK 47295 114 METREX 60104 115 MICROFLEX CT-133-L 116 MICROFLEX CT-133-M 117 MICROFLEX CT-133-S 118 MICROFLEX 119 DGP-350-L MASKS, EARLOOP PROCEDURE, ORANGE, FLUIDSHIELD, KIMBERLY CLARK, 47107, 40/BX GLOVES, DENTAL P/F NITRILE N/S, SMALL, SAFESKIN, KIMBERLY CLARK, 53431, 100/BX MASKS, CARE-BEAR PROCEDURE, EARLOOP, KIMBERLY CLARK, 47295, 50/BX DISINFECTANTS, CAVICIDE, GALLON, METREX, 060104, EACH GLOVES, LATEX POWDER N/S, LARGE BLUE, COLOR TOUCH, MICROFLEX, CT-133L, 100/BX GLOVES, LATEX POWDER N/S, MEDIUM GREEN, COLOR TOUCH, MICROFLEX, CT-133M, 100/BX GLOVES, LATEX POWDER N/S, SMALL PINK, COLOR TOUCH, MICROFLEX, CT-133-S, 100/BX GLOVES, LATEX P/F TEXT N/S, LARGE, DIAMOND GRIP PLUS, MICROFLEX, DGP-350-L, 100/BX RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 95 MICROFLEX DGP-350-M 120 MICROFLEX DGP-350-S 121 MICROFLEX DGP-350-XS 122 MICROFLEX DGP-350-XL 123 MICROFLEX EV-2050-S 124 MICROFLEX EV-2050-XS 125 MICROFLEX MF-300-M 126 MICROFLEX MF-300-S 127 MIDWEST DENTAL CORP 780014 128 MIDWEST DENTAL CORP 780045 GLOVES, LATEX P/F TEXT N/S, MEDIUM, DIAMOND GRIP PLUS, MICROFLEX, DGP-350-M, 100/BX GLOVES, LATEX P/F TEXT N/S, SMALL, DIAMOND GRIP PLUS, MICROFLEX, DGP-350-S, 100/BX GLOVES, LATEX P/F TEXT N/S, X-SMALL, DIAMOND GRIP PLUS, MICROFLEX, DGP-350-XS, 100/BX GLOVES, LATEX P/F TEXT N/S, X-LARGE, DIAMOND GRIP PLUS, MICROFLEX, DGP-350-XL, 100/BX GLOVES, LATEX P/F TEXT N/S, SMALL, EVOLUTION ONE, MICROFLEX, EV2050-S, 100/BX GLOVES, LATEX P/F TEXT N/S, X-SMALL, EVOLUTION ONE, MICROFLEX, EV2050-XS, 100/BX GLOVES, LATEX P/F TEXT N/S, SMALL, DIAMOND GRIP, MICROFLEX, MF-300S, 100/BX GLOVES, LATEX P/F TEXT N/S, MEDIUM, DIAMOND GRIP, MICROFLEX, MF-300M, 100/BX Hand Pieces, Quiet-Air L Hp F/O W/Power, F/O, Midwest, 780014, Ea 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Hand Pieces, Tradition L F/O Hp W/Power, Midwest, 780045, Ea 129 0.00 MIDWEST DENTAL CORP 790044 Hand Pieces, Tradition 4-Hole, Hi-Speed, Midwest, 790044, Ea 130 0.00 MIDWEST DENTAL CORP 790045 Hand Pieces, Tradition Fiber Op, 5-Hole, Midwest, 790045, Ea 389105 CARBIDE BURS, RIGHT ANGLE, 4 ROUND, MIDWEST, 389105, 10/PK 389259 CARBIDE BURS, FRICTION GRIP, 1557 STRAIGHT DOME CROSSCUT, MIDWEST, 389259, 10/PK 131 0.00 MIDWEST DENTAL CORP 132 MIDWEST DENTAL CORP 133 MILTEX 134 0.00 DM303RA Finishing & Polishing, Mandrel Ra #303, Miltex, Dm303Ra, 12/Pk RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 Page 96 NICEPK Q85084 135 ORALBL 75035355 136 PALMERO SALES 10DIS 137 PALMERO SALES 1950 138 PALMERO SALES 60DIS 139 PASCAL CO INC 11-390 140 PASCAL CO INC 26-110 141 PATTERSON 78009 142 PATTERSON 122210 143 PATTERSON 143112 144 PATTERSON 05 21362 145 PATTERSON 083-8367 146 PATTERSON PATTERSON SYRINGE COVERS, DISPOSABLE, PALMERO, 1950, 100/BX DISINFECTANTS, DISCIDE ULTRA, 6"X6.75" TOWELETTES, PALMERO, 60DIS, 160/PK FLOURIDE RINSE KIT, 60:60 IN-OFFICE CONCENTRATE, TROPICAL BLAST, 4 PART A & 1 PART B, PASCAL, 11-390, CS INSTRUMENT, CORD PACKER, R11 SERRATED DOUBLE END, PASCAL, 26110, EACH CARBIDE BURS, FRICTION GRIP, SURGICAL, 702 CC TPR FISS, PATTERSON, 78009, 5/PK AUTOCLAVE FILM, 2" WIDE, STEAM ONLY, PATTERSON, 122210, 100'/BX PROPHY ANGLES, DISPOSABLE, SOFT CUP GREY, PATTERSON, 0850149, 125/BX ULTRASONIC CLEANING SOLUTION, GENERAL PURPOSE, SUPER CONCENTRATE, PATTERSON, 0903005, 16OZ BOTTLE INSTRUMENT, AMALGAM CARRIER, STEEL DE MEDIUM/LARGE, PATTERSON, 0838367, EACH 084-9265 X-RAY FILM, CD58, SIZE 2 PAPER, PATTERSON, 0849265, 144/BX 085-0503 CHAIR COVER SLEEVE, CLEAR PLASTIC, 24" LONG, PATTERSON, 0850503, 225/BX 147 148 Infection Control Products, Sani-Cloth Plus X-Lg, 8"X14", Q85084, 65/Pk (replaces #2672940) Preventive Products, Advantage Plus Toothbrush, 40 Soft, 75035355, 12/Bx (replaces #3251428) DISINFECTANTS, DISCIDE ULTRA, 10.5"X10.5" TOWELETTES, PALMERO, 10DIS, 60/PK RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 97 150 LIGHT HANDLE COVER, T-STYLE, 4"X5.75", CLEAR PLASTIC, PATTERSON, 0850545, 500/BX TRAY COVER SLEEVES, SIZE B, 10.5"X14", PATTERSON, 0850669, 500/BX 151 UNIVERSAL COVER SHEETS, 4"X6", BLUE, PERFORATED ROLL, PATTERSON, 085-0768, 1200/BX PATTERSON 085-0545 149 PATTERSON PATTERSON PATTERSON 085-0669 085-0768 085-1725 GLOVES, VINYL P/F, SMALL, PATTERSON, 085-1725, 100/BX 085-1733 GLOVES, VINYL P/F, MEDIUM, PATTERSON, 0851733, 100/BX 085-1741 GLOVES, VINYL P/F, LARGE, PATTERSON, 0851741, 100/BX 152 0.00 0.00 0.00 PATTERSON 153 PATTERSON 154 PATTERSON 085-1808 155 PATTERSON 088-6614 156 PATTERSON 089-4303 157 PATTERSON 089-9641 158 PATTERSON 089-9666 159 PATTERSON 088-7687 160 PATTERSON 10-0101 161 PATTERSON 162 0.00 10713 HEAD REST COVER, 9.5"X11", CLEAR PLASTIC, PATTERSON, 0851808, 250/PK MATRIX BAND, TOFFLEMIRE, SIZE #1 .0015 GAUGE, PATTERSON, 0886614, 12/PK SURGICAL ASPIRATOR TIPS, REGULAR 1/8" TIP, WHITE, PATTERSON, 089-4303, 25/PK SUTURES, 4-0 BLACK BRAIDED SILK 18", DS-18 3/8" CIRCLE REVERSE NEEDLE, CP66, PATTERSON, 0899641, 12/BX SUTURES, 3-0 BLACK BRAIDED SILK 18", DS-18 3/8" CIRCLE REVERSE NEEDLE, CP72, PATTERSON, 0899666, 12/BX INSTRUMENT, COTTON PLIER, COLLEGE 317, SERRATED, PATTERSON, 0887687, EA XRAY FILM MOUNTS, GRAY CARDBOARD, 1H #2, PATTERSON, 10-0101, 100/PK MASKS, MOLDED SURGICAL CUP, BLUE, PATTERSON, 10713, 50/BX RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 98 PATTERSON 689265 163 PATTERSON 7000861 164 PATTERSON 78005 165 PATTERSON 083-8169 166 PATTERSON 917463 167 PATTERSON 9575930 ALGITEC ALGINATE, DUST-FREE, REGULAR SET, PATTERSON, 689265, 1 LB.CAN HVE ASPIRATOR TIPS, NON-SLOTTED ENDS, DISPOSABLE, PATTERSON, 0894287, 50/BG CARBIDE BURS, FRICTION GRIP, SURGICAL, 557 CC CYL FISS, PATTERSON, 78005, 5/PK STERILIZATION POUCHES, BUR SIZE, 2 1/4"X4", SELF SEAL, PATTERSON, 083-8169, 200/BX PATIENT BIBS, ECONOBACK TOWEL, BLUE, 2 PLY PAPER & 1 PLY POLY, PATTERSON, 917463, 500/CS 0.00 0.00 0.00 0.00 0.00 DENTURE BOX, BLUE, PATTERSON, 9575930, 12/PK 168 0.00 PATTERSON 19063 169 PATTERSON 05 21120 170 PATTERSON 089-0376 171 PDI PROFESSIONAL DISPOSABLES Q24272 172 PDI PROFESSIONAL DISPOSABLES SANHBL 173 PDI PROFESSIONAL DISPOSABLES SANL 174 Pinnacle 2200 GLOVES, LATEX POWDERED N/S, SIZE 7 1/2, HAND SPECIFIC, PATTERSON, 19063, 50PR/BX EVACUATION CLEANER, DEODORIZER, SUPER CONCENTRATED LIQUID, PATTERSON, 05 21120, 32OZ BOTTLE ETCHING GEL, 40% PHOSPHORIC ACID, 4-5ML SYRINGE PACK W/ TIPS, PATTERSON, 0890376, PK DISINFECTANTS, SANI-CLOTH HB, XLARGE, GERMICIDAL WIPE, PDI PROFESSIONAL, Q24272, 60/PK DISINFECTANTS, SANI-CLOTH HB, LARGE, GERMICIDAL WIPE, PDI PROFESSIONAL, SANHBL, 160/PK DISINFECTANTS, SANI-CLOTH PLUS, LARGE, GERMICIDAL WIPE, PDI PROFESSIONAL, SANL, 160/PK 0.00 0.00 0.00 0.00 0.00 0.00 Evac-U-Trap Filter, #2200, Pinnacle 2200, 12/Box 175 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 99 PINNACLE PRODUCTS INC 3800 176 PINNACLE PRODUCTS INC 3825 177 PINNACLE PRODUCTS INC 3850-XL 178 PINNACLE PRODUCTS INC 4575 179 SAFE-WAVE 66150 180 SAFE-WAVE 44150 181 SCHEIN 2015005Z CHAIR COVER SLEEVE, CLEAR PLASTIC, 27.5"W X24"L, PINNACLE, 3800, 225/BX CHAIR COVER SLEEVE, CLEAR PLASTIC, 32"W X32"L, PINNACLE, 3825, 200/BX CHAIR COVER SLEEVE, CLEAR PLASTIC, 29"W X80"L, PINNACLE, 3850-XL, 225/BX CURING LIGHT SHIELDS, VISION SAVER, ORANGE, DISPOSABLE, PINNACLE, 4575, 400/BX SYRINGE TIPS, SPREE, DISPOSABLE, NO ADAPTER NEEDED, SAFE-WAVE, 66150, 150/PK SYRINGE TIPS, SAFE-TIPS EZ, DISPOSABLE, NO ADAPTER NEEDED, SAFE-WAVE, 44150, 150/PK 0.00 0.00 0.00 0.00 0.00 Abrasives Lab, Lab Pumice Medium, HSI, 2015005Z, 5Lb/Ea 182 0.00 SCHEIN 51008 Alloys, Stratosphere Caps Set, 2 Spill, HSI, 51008, 500/Jr 1007638 Crown & Bridge Products, Copper Bands W/Chest Hard, Asst, HSI, 1007638, 100/Bx 183 0.00 SCHEIN 184 SCHEIN 1008005 Dental Hand Instruments, Band Remover, Baade, HSI, 1008005, Ea 1006436 Dental Hand Instruments, Elevator Cryer #34S, HSI, 1006436, Ea 185 SCHEIN 186 SCHEIN 1006640PLC 187 SCHEIN #4-P/S-C/S 188 SCHEIN SCHEIN Dental Hand Instruments, Mirror Disposable Dental, HSI, 1006640Plc, 72/Bx Dental Hand Instruments, Mirror Plane Cone Socket, #4, HSI, #4-P/S-C/S, 12/Bx 100-4888 Dental Hand Instruments, Periosteal Elevator Molt, HSI, 100-4888, Ea 1008966 Dental Hand Instruments, Pliers 114 Standard, Johnson, HSI, 1008966, Ea 189 190 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 100 SCHEIN SDS-TT-AS Dental Hand Instruments, Tape N Tell Kit, 8/Rl 191 0.00 SCHEIN 741 192 SCHEIN WESCEBL Dental Small Equipment, Ultrasonic Cleaner W/Digital, Timer, HSI, 741, Ea (replaces #1006879) Disposables, Dri-Gard Towel 2Ply+Poly, Blue, HSI, 500/Ca 193 0.00 SCHEIN WESCELV Disposables, Dri-Gard Towel 2Ply+Poly, Lavender, HSI, 500/Ca 194 0.00 SCHEIN WESCEPE Disposables, Dri-Gard Towel 2Ply+Poly, Peach, HSI, 500/Ca 1002550 Disposables, Tray Cover 11"X17.25", White, HSI, 1002550, 1000/Bx 1008128 Disposables, Tray Cover 9"X13.5", White, HSI, 1008128, 1000/Bx 1000934 Disposables, Tray Covers 8.5"X12.25", White, HSI, 1000934, 1000/Bx 1029023 Evacuation Products, Aspirator Tips Green, 1/4"D, HSI, 1029023, 25/Pk 195 0.00 SCHEIN 196 SCHEIN 197 SCHEIN 198 SCHEIN 199 SCHEIN 7001423 Evacuation Products, Evacuator Tip Vented, HSI, 7001423, 50/Bag 1005848 Hand Pieces, Contra Angle 20,000 Rpm, #150, HSI, 1005848, Ea 1002037 Hand Pieces, Lubricant Oil For Master, #450, HSI, 1002037, 1Oz/Bt 200 0.00 0.00 0.00 0.00 0.00 SCHEIN 201 SCHEIN 202 SCHEIN FPSCHEIN108 203 SCHEIN SCHEIN Impression Materials & Access, Impression Tray #4 Med/L, HSI, Fp-Schein109, 12/Bag FPSCHEIN110 Impression Materials & Access, Impression Tray #5 /U, HSI, FpSchein110, 12/Bag FPSCHEIN112 Impression Materials & Access, Impression Tray #7, HSI, FpSchein112, 12/Bag 205 SCHEIN Impression Materials & Access, Impression Tray #3 Med/U, HSI, Fp-Schein108, 12/Bag FPSCHEIN109 204 206 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 Page 101 SCHEIN FPSCHEIN113 Impression Materials & Access, Impression Tray #8, HSI, FpSchein113, 12/Bag 1005870 Infection Control Products, Disp. Cover Gown-Blue, Med/Lg, HSI, 1005870, 10/Pk 207 SCHEIN 208 SCHEIN 1043809 209 SCHEIN 1043730 210 SCHEIN 1042849 211 SCHEIN 1009972 212 SCHEIN 10-6760 213 SCHEIN 50036970 214 SCHEIN 1009860 215 SCHEIN 1008356 216 SCHEIN FCS WQ150T4/ CL/2 Large Equipment Accessories, Bulb Quartz #Q150 , 25V 150, HSI, Wq150 Ea FPSCHEIN 104 Orthodontic Products, Ortho Glass Ionomer, 15Gm, HSI, Fpschein104, Ea 28221 Preventive Products, Acclean Toothbrush Youth, 28 Tuft, HSI, 28221, 72/Bx 220 SCHEIN 221 Infection Control Products, Schein Face Masks Molded, Blue, HSI, 1008356, 50/Bx Large Equipment Accessories, Bulb Overhead A-Dec, 24V 150, HSI, Ea 219 SCHEIN Infection Control Products, Schein Air/Water Cover, 2.5"X10, HSI, 1009860, 500/Bx Large Equipment Accessories, Bulb #150T4/24V, 24V150W, HSI, Ea 218 SCHEIN Infection Control Products, Maxizyme Tabs, HSI, 50036970, 64/Bx DZE 217 SCHEIN Infection Control Products, Ear loop Face Masks, Blue, HSI, 1043809, 50/Bx (replaces #1006004) Infection Control Products, Ear loop Face Masks, Pink, HSI, 1043730, 50/Bx (replaces #1005937) Infection Control Products, Ear loop Face Masks, Yellow, HSI, 1042849, 50/Bx (replaces #1015420) Infection Control Products, Lab Gowns Disposable White, Sm/Med, HSI, 1009972, 10/Pk Infection Control Products, Maxicide W/Activator 2.5%, 28 Day, HSI, 10-6760, Gallon RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 102 SCHEIN 140210 Preventive Products, Disposable Prophy Angle, Soft, HSI, 140210, 100/Bx FPSCHEIN 060 Restorative Materials, Flowable Composite Kit, Intro, HSI, Fpschein060, 4/Bx FPSCHEIN 121 Restorative Materials, Flowable Composite Syring, Tips, HSI, Fpschein121, 100/Bag 222 SCHEIN 223 SCHEIN 224 SCHEIN 206100250100 0.00 0.00 Rotary Instruments, Bur Carbide, 1/4, HSI, 206100250100, 10/Pk 225 0.00 SCHEIN 206100251500 226 SCHEIN 206100250400 Rotary Instruments, Bur Carbide, 1157, HSI, 206100251500, 10/Pk 0.00 Rotary Instruments, Bur Carbide, 2, HSI, 206100250400, 10/Pk 227 0.00 SCHEIN S20610025060 Rotary Instruments, Bur Carbide, 4, HSI, S20610025060, 10/Pk 228 0.00 SCHEIN 206100252900 Rotary Instruments, Bur Carbide, 557, HSI, 206100252900, 10/Pk 206100250200 Rotary Instruments, Burs Carbide, 1/2, HSI, 206100250200, 10/Pk 206100182100 Rotary Instruments, Burs Carbide 245, HSI, 206100182100, 100/Pk 206100181800 Rotary Instruments, Burs Carbide 330, HSI, 206100181800, 100/Pk 9897 Rotary Instruments, Burs Carbide Lab Lathe Re, A 3/8, HSI, 9897, Ea 9898 Rotary Instruments, Burs Carbide Lab Lathe Re, B 3/8, HSI, 9898, Ea 229 0.00 SCHEIN 230 SCHEIN 231 SCHEIN 232 SCHEIN 233 SCHEIN 234 SCHEIN 9899 235 SCHEIN SCHEIN Rotary Instruments, Burs Carbide Lab Lathe Re, C 3/8, HSI, 9899, Ea 9900 Rotary Instruments, Burs Carbide Lab Lathe Re, D 3/8, HSI, 9900, Ea 206100260100 Rotary Instruments, Burs Carbide Ra, 1/2, HSI, 206100260100, 10/Pk 236 237 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Page 103 SCHEIN 206100260300 Rotary Instruments, Burs Carbide Ra, 2, HSI, 206100260300, 10/Pk 206100260400 Rotary Instruments, Burs Carbide Ra, 4, HSI, 206100260400, 10/Pk 238 SCHEIN 239 SCHEIN HSI6008-15 240 SCHEIN 100-6120 241 SCHEIN 27270-60 242 SCHEIN 5226 X-Ray Products, KlearVue Cardboard Mount, 8H 6V 2, HSI, 5226, 100/Bx 01-A1400 Anesthetic, Septocaine Articaine 4%, Septodont 01A1400, 50/Box 8881-400058 Needle, Monoject 27L Plastic Hub #400, Sherwood 8881400058, 100/Box 8881-400074 Needle, Monoject 30S Plastic Hub #400, Sherwood 8881400074, 100/Box 8881-401056 Needle, Monoject 27L Metal Hub #401, Sherwood 8881401056, 100/Box 245 Sherwood 246 Sherwood 247 Sherwood 248 Star 260296 249 STAR X-Ray Products, Automatic A&B Developer &, 2Gal, HSI, 27270-60, 4/Ca X-Ray Products, Dx-58 Vinyl Packet Size 2, 1 Film, HSI, Umgd58, 150/Bx 244 Septodont Wound Closure, Suture Plain Gut C-9 3/0, 18", HSI, 1006120, 12/Bx UMGD58 243 SCHEIN Surgical Products, Scalpels Disposable, #15, HSI, HSI6008-15, 10/Bx 262052 Hand piece Turbine, Star 430 Auto chuck LUBE TYPE, Star 260296, Each 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 Hand Pieces, 430K Hp Vortex-No Swivel, 4Hole, Star, 262052, Ea 250 0.00 STAR 259005 251 STAR 264073 Hand Pieces, Ball Bearing Latch Contra, Angle, Star, 259005, Ea 0.00 Hand Pieces, MotorTo-Angle Adaptor, Star, 264073, Ea 252 0.00 STAR 260144 Hand Pieces, Swivel Attachment 4 Line, Star, 260144, Ea 253 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 Page 104 STAR 262262 Hand Pieces, Titan 3 Motor 20000 Rpm, Star, 262262, Ea 254 0.00 STAR 262261 Hand Pieces, Titan 3 Motor 5000 Rpm, Star, 262261, Ea 255 0.00 STAR 258443 Hand Pieces, Titan-2 Plus Torque Multi, Star, 258443, Ea 264151 Hand Pieces, Titan3 Lube free Auto chuck, Angle, Star, 264151, Ea 95029-000 DISINFECTANTS, LYSOL IC SPRAY W/ CONTROL FLOW, SULTAN CHEMISTS, 95029-000, 19OZ EACH 7510 Cements - Liners Bases, Vitrebond Intro Pkg, Package, 7510, Ea 256 0.00 STAR 257 SULTAN CHEMISTS 258 THREEM 259 THREEM 12622 Preventive Products, Clinpro Sealant Refill Pk, 12622, 6Ml 12627 Preventive Products, Clinpro Sealant Refill Pkg, Syr1.2M, 12627, Ea 7543 Restorative Materials, Adper Scotchbond M/P Adhesive, #7543, 7543, 8Ml/Bt 7540S Restorative Materials, Adper Scotchbond M/P Sq, Adhesive, 7540S, Ea 260 0.00 0.00 0.00 THREEM 261 THREEM 262 THREEM 263 THREEM 3303L 0.00 0.00 0.00 Restorative Materials, Vitremer Liquid, 3303L, 8Ml/Bt 264 0.00 TILLOT 6103 Gloves, Formula 1 Latex Glove, 6103, 100/Bx 265 0.00 Tillotson 6612 266 TRONEX WARNER LAMBERT Glove, Plus Powder Free Small, Tillotson Healthcare 6612, 100/Box 5540B Infection Control Products, Gown One Size Fits All, Blue, 5540B, 50/Ca 42750 MOUTHWASH, LISTERINE COOL MINT, GALLON BOTTLE, WARNER LAMBERT, 42750, 2/CS 267 268 0.00 RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 Page 105 WARNER LAMBERT 42755 269 Whaledent UC300115B 270 WHALEDENT C7964 271 Young 177001 Rubber Dam Frame, 4 1/8 x 3 5/8 Chrome Plated Brass, Young Dental 177001, Each 77225 SURGICAL ASPIRATOR TIPS, SURG-O-VAC II, 1/4" TIP, GREEN, YOUNG DENTAL, 077225, 25/PK 272 YOUNG DENTAL 273 MOUTHWASH, LISTERINE COOL MINT, 1.5L BOTTLES, WARNER LAMBERT, 42755, 6/CS Dental Small Equipment, Biosonic Uc300 Ultrasonic, Cleaner, Uc300115B, Ea CURING LIGHT, COLTOLUX 75, 75 WATT HALOGEN WITH 8MM TIP, WHALEDENT, C7964, EACH RFP No. 10-475-000-J Medical and Dental Supplies 0.00 0.00 0.00 0.00 0.00 Page 106 Quarterly Contract Sales Summary Report Contractor Name: FEID#: Contact Person: Phone Number: Contract Title: Contract #: Email: Fax or Email form to: Donna Smith, 850-414-6122 or Donna.Smith@dms.myflorida.com CALENDAR QUARTER: Check One Quarterly Period Ending March 31st Quarterly Period Ending June 30th Quarterly Period Ending September 30th Quarterly Period Ending December 31st ENTITY STATE AGENCIES: Report dollar amount sold to all State Agencies. List each Agency separately. POLITICAL SUBDIVISIONS: Report dollar amount sold to other Political Subdivisions [including but not limited to, Counties, Cities, Schools, Universities, Colleges and Utilities]. List each entity separately. GRAND TOTAL: TOTAL DOLLARS $ $ $ Under penalties of perjury, I declare that this is a true and accurate report of all sales due under the terms and conditions of this state term contract for the specified quarterly reporting period. AUTHORIZED TYPED SIGNATURE: AUTHORIZED ELECTRONIC SIGNATURE: Notes: 1) A quarterly report is required even if there are no sales for the specified quarter; please enter zero dollars where applicable. RFP No. 10-475-000-J Medical and Dental Supplies Page 107 2) This form is for the reporting of quarterly sales only. It is not related to reporting and payment of vendor transaction fees. 3) To enter electronic signature, click text box, click “Insert” (on tool bar), select “picture”, and select picture type to paste or enter signature. 4) For information concerning the use of this form, please contact the Contract Administrator named above. (Rev. 3/05/09) CONTRACT This Contract, effective the last date signed below, is by and between the State of Florida, Department of Management Services (“Department”), an agency of the State of Florida with offices at 4050 Esplanade Way, Tallahassee, Florida 32399-0950, and the entity identified below as Contractor (“Contractor”).The Contractor responded to the Department’s Request for Proposal No. 10-475-000-J for Medical and Dental Supplies. The Department has determined to accept the Contractor’s response and to enter into this Contract in accordance with the terms and conditions of the solicitation. Accordingly, and in consideration of the mutual promises contained in the Contract documents, the Department and the Contractor do hereby enter into this Contract, which is a state term contract authorized by section 287.042(2)(a) of the Florida Statutes (2001). The term of the Contract is 36 months from the effective date. The Contract consists of the following documents, which, in case of conflict, shall have priority in the order listed, and which are hereby incorporated as if fully set forth: Any written amendments to the Contract Technical Specifications Special Contract Conditions General Contract Conditions (PUR 1000) Special Instructions to Respondents General Instructions to Respondents (PUR 1001) This document Any Purchase Order under the Contract Contractor’s response ___________________________________________ State of Florida, Date Department of Management Services By: Linda H. South, Secretary Contractor Name: Street Address or P.O. Box: City, State, Zip: (Seal) _______________________________________________ By: Date Its (Title): _________________________________ RFP No. 10-475-000-J Medical and Dental Supplies Page 108 Approved as to form and legality by the General Counsel’s Office ______________________________________ Print name: __________________________ RFP No. 10-475-000-J Medical and Dental Supplies Date: ____________________ Page 109