CGA15-Summary
advertisement

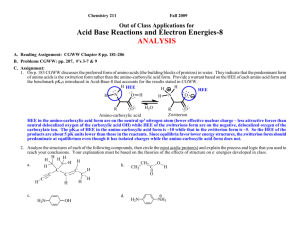
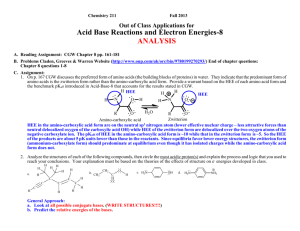
Chemistry 211 2007 Equilibrium Controlled Reactions: Class Group Activity #15: Carbonyl Reactions – 2 SUMMARY OF CLASS DISCUSSION C. Exploration: Mechanisms of Addition Reactions 1. Most complex reactions occur through a sequence of simple reaction steps as illustrated in Activity 14 part D. 2. Use the proposed analytical process outlined below to help you write a reasonable reaction mechanism (sequence of simple reaction steps illustrated with curved arrows) for addition reaction d. reproduced below. These reactions are equilibrium reactions (fast in both the forward and reverse directions). So, it is important that energies of the highest energy electrons in all intermediate products in your mechanisms be kept as low as possible. One very high energy intermediate will essentially stop the progress of the reaction along that path. One way to keep the energy low in creating a proposed mechanism is to use the highest energy electrons in each reaction step to form a bond in moving to the next intermediate. Recall that forming a bond to the HEE’s will cause a relatively large decrease in energy. This energy decrease will help offset any energy increases when other bonds are broken. O d. CH2 C CH3 CH2 O + CH3 H CH2 CH2 H O OH C H2O H CH3 CH2 O H C CH CH2 CH3 CH2 C H Propose Analytical Process for Finding a Relatively Low Energy Pathway from Reactants to Products a. Find Initial HEE’s: Identify the highest energy e-'s at the beginning of the reaction. Don't forget to consider the solvent and catalyst. (structures over and under the reaction arrows) Indicate reactant HEE’s on structures above and explain how they were chosen below. The reaction conditions are strongly basic with the highest energy e- pair on HO-. Hydroxide ion has its lone pair e-'s on a negative sp3 oxygen. The only other lone pairs of e-'s are on the solvent, H2O, with the lone pair e-'s on a neutral sp3 oxygen and the carbonyl oxygen, which is a neutral sp2 oxygen. Both the charge and the hybridization of the carbonyl oxygen indicate that its lone pair e-'s will have lower energy than those on the hydroxide oxygen because of both stronger e--nuclear attraction due to higher scharacter (hybridization effect) and less e--e- repulsion due to less excess e- density (less negative net charge). At the same time the lone pair e-'s on the neutral water oxygen atoms will have lower energy than those of the hydroxide oxygen because of less e--erepulsion due to less excess e- density (less negative ionic charge). So the order of lone pair e- energies should be HO- highest, then the H2O and the carbonyl oxygen should be lowest. Therefore, hydroxide ion, holding the highest energy e- pair, should be the most likely species to react in the first step since its energy will be lowered more than the other lower energy e-'s. b. Determine bonds made and broken: Identify the bonds that need to be made and broken to convert the reactants to the products. (Identified previously for rxn d.) d. C-O -bond broken CH3 CH2 H C-C -bond made O C C H O + H CH3 CH2 H - C C H H OH H2O C-H -bond broken H O-H -bond made CH3 CH2 O H C CH CH2 CH3 CH2 O C H CGA # 15 Complex Equilibrium Controlled Reactions 2 The overall reaction requires the breakage of a C-H bond on the -carbon of one butanal (butyraldehyde) molecule, formation of a -bond between a hydrogen and the carbonyl oxygen of a second butanal molecule, breakage of the carbonyl π-bond and the formation of a -bond between the -carbon atom of the butanal molecule that lost a hydrogen and the carbonyl carbon of the other butanal molecule. c. Use HEE’s to make needed bond changes & draw resulting structures: As we discussed in class, any concerted (one-step) mechanism would require a four centered transition state or a termolecular (three different molecules) transition state. Both possibilities are unlikely so: The best choices for the first step in this reaction seem to be either the deprotonation of the -carbon atom of butanal by HO - , or a direct nucleophilic addition of HO - to the carbonyl carbon. Thus, hydroxide can lower the energy of its electrons by forming a bond to either a proton (acting as a base) or to a carbon atom (acting as a nucleophile). Let's consider each possibility! (1.) Deprotonation of the of the -Carbon of Butanal: As we discussed in class, the -hydrogen is the most acidic proton available for the base because the conjugate base is delocalized by resonance onto the adjacent carbonyl oxygen. ..O.. H + CH3 CH2 - C C H .. -... . ..OH ..O.. H CH3 CH2 CH C .. H2O .. + H .. .. O ..CH3 CH2 CH C H Accomplishments: - Breaks the C-H -bond required in the reaction. - Creates an O-H -bond and transfers the lone pair of e-’s to the -carbon of the acetaldehyde creating a delocalized anion. Potential problems: - The new O-H bond formed is not present in the products. (2.) Direct addition of hydroxide to the carbonyl carbon: The addition occurs at the carbonyl carbon rather than the oxygen because the newly generated lone pair of e-’s can be transferred to the oxygen (equivalent effective nuclear charge) rather than to the carbon. . . .. ..O ..O.. -... ...OH + CH 3 CH 2 CH 2 C H CH 3 CH 2 CH 2 C H . ..O . H Accomplishments: - Breaks the C=O π-bond required in the reaction. - Creates a C-O -bond and transfers the negative charge to another sp3 oxygen. Potential problems: - The new C-O bond is in the products. CGA # 15 Complex Equilibrium Controlled Reactions 3 ANALYSIS OF THE REACTIONS (1.) & (2.) Overall Change in Structure: -In step (1.), an H-O -bond is formed while a C-H -bond is broken and the lone pair of e-’s is moved from a negative sp3 oxygen to a delocalized ion. -In step b., a C-O -bond is formed while a C-O π-bond is broken and a lone pair of e-’s is moved from one negative sp3 oxygen to another. Change in Energy Due to Structural Changes: -The net change in energy in step (1.) is less favorable than step (2.) The change in position of the highest energy pair of e-’s is unfavorable. -Step (2.) is favorable because the movement of the highest energy pair of e-’s causes little or no change in free energy of the highest e-'s. So, this reaction is close to energy neutral. However it is reversible and does not obviously lead to any of the products. d. Find new HEE’s & Compare Energies with Initial HEE’s: Identify the HEE's in your intermediate structures. If you are evaluating more than one possibility, identify the highest energy e-'s in each set of intermediate structures. Compare the energies of each group of highest energy e-'s to that of the highest energy e-'s at the beginning of the reaction. Any set of intermediates is acceptable if the HEE's are no more than ~5 pK units higher in energy than those at the beginning of the reaction. If there is more than one acceptable intermediate, choose the one with the lowest energy HEE's. Most likely Course of the Reaction: -- Step (1.) is sufficiently favorable, increase in e- energy of < pK 5 units (-carbon pKa = ~20 vs. H2O pKa = ~16), that a significant amount of the -anion can form and this step does accomplish one of the key bond breakages that is required ( C-H). Since step (2.) is reversible then the major course of the overall reaction is through step a. e. Use New HEE’s to make needed bond changes & draw resulting structures: (Same process as in c.) Now the highest energy pair of e-'s is on the -carbon of the "enolate ion" of butanal which was formed in step (1.) Thus, this ion is likely to be involved in the next step. As before it can either form a bond to a proton (act as a base) or to a carbon atom (act as a nucleophile). If it acts as a base, it will reverse step (1.), so the most likely reaction of the "enolate ion" that will lead toward the products is step (3.), nucleophilic addition of the ion to the carbonyl carbon of a second butanal molecule. (see next page) (3.) Addition of -anion formed in step b. to the carbonyl carbon. ..O.. CH 3 CH 2 CH .. - C . . .. - . ..O ..O.. H + CH 3 CH 2 CH 2 C H CH 3 CH 2 CH 2 CH 3 H C CH ..O.. C H CH 2 Overall Change in Structure: -- Accomplishes the breakage of the C=O π-bond required in the reaction. -- Creates the new C-C -bond required in the reaction and transfers the lone pair of e-’s to a negative sp3 oxygen. CGA # 15 Complex Equilibrium Controlled Reactions 4 f. Continue Step d & e: Find new HEE’s & Compare Energies with Initial HEE’s:Most likely Course of the Reaction: Change in Energy Due to Structural Changes: -- This step is a favorable process. The highest energy pair of e-’s is moved from a higher energy delocalized position to a lower energy negative sp3 oxygen position. -- The lowering of the energy of the lone pair e-’s and changes in bonding are both favorable so step (3.) is the a very reasonable second step in the reaction mechanism. Use New HEE’s to make needed bond changes & draw resulting structures: (Same process as in c.) After step (3.), the highest energy e- pair is on the negative sp3 oxygen created by the addition to the carbonyl carbon. Thus, this oxygen is likely to be involved in the next reaction step. To complete the formation of products and regenerate the HO - catalyst, the next step is likely to be the proton transfer indicate in step d. (4.) Protonation of the Addition Intermediate formed in step c. to Produce the Final Product and Regenerate the Base Catalyst. . . .. - ..O CH 3 CH 2 CH 2 CH 3 H C CH CH 2 ..O.. C H + .. H ..O .. ..O H CH 3 CH 2 CH 2 CH 3 H H C CH ..O.. C H -.. + ..OH .. CH 2 Overall Change in Structure: -- Creates the new O-H -bond required in the reaction and transfers the highest energy pair of e-’s to another negative sp3 oxygen regenerating the HO - catalyst consumed in step (1.) Change in Energy Due to Structural Changes: -- This reaction produces little or no change in free energy since the highest energy pair of e-’s is moved from one negative sp3 oxygen to another negative sp3 oxygen and there is no net change in the types of bonds. CGA # 15 Complex Equilibrium Controlled Reactions 5 Since this reaction mechanism requires a base catalyst in the first step, it is considered to be a base catalyzed reaction mechanism. So, it is called: BASE CATALYZED NUCLEOPHILIC ADDITION TO A CARBONYL GENERAL FACTORS THAT HELP IN IDENTIFYING WHEN BASE CATALYZED ADDITION IS LIKELY Weakly or strongly basic conditions where the nucleophile must be initially deprotonated. (Note that the - is moved from one molecule to another and no + charges or additional charges are formed.) Nu + H - :B - Nu: + H B The above step is the key to whether or not this process is likely to be favorable. If the newly formed e- pair on the nucleophile (Nu: - ) is more than about 5 pKa units higher in energy than that on the base ( - :B), then too little Nu: - will be formed at equilibrium to keep the reaction proceeding at a sufficient rate to be practical. The last two steps are generally favorable or only very slightly uphill in energy. O O - Nu : C + - C Nu O - C Nu O + H B C Nu H + - B: