211-09OCAAnal-AB
advertisement

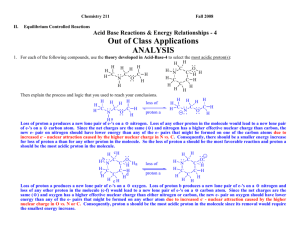
Chemistry 211 Fall 2009 Out of Class Applications for Acid Base Reactions and Electron Energies-8 ANALYSIS A. Reading Assignment: CGWW Chapter 8 pp. 181-206 B. Problems CGWW: pp. 207, #’s 3-7 & 9 C. Assignment: 1. On p. 183 CGWW discusses the preferred form of amino acids (the building blocks of proteins) in water. They indicate that the predominant form of amino acids is the zwitterion form rather than the amino-carboxylic acid form. Provide a warrant based on the HEE of each amino acid form and the benchmark pKas introduced in Acid-Base-8 that accounts for the results stated in CGWW. H HEE H H HEE N O O N H H H H2 O O R O R Zwitterion Amino-carboxylic acid HEE in the amino-carboxylic acid form are on the neutral sp3 nitrogen atom (lower effective nuclear charge – less attractive forces than neutral delocalized oxygen of the carboxylic acid OH) while HEE of the zwitterions form are on the negative, delocalized oxygen of the carboxylate ion. The pKaH of HEE in the amino-carboxylic acid form is ~10 while that in the zwitterion form is ~5. So the HEE of the products are about 5 pK units lower than those in the reactants. Since equilibria favor lower energy structures, the zwitterion form should predominate at equilibrium even though it has isolated charges while the amino-carboxylic acid form does not. 2. Analyze the structures of each of the following compounds, then circle the most acidic proton(s) and explain the process and logic that you used to reach your conclusions. Your explanation must be based on the theories of the effects of structure on e- energies developed in class. H H H H H H CH2 O a. b. H CH3 C H H C C O C C C H C H H H c. d. H2N OH + H2N NH3 2 Acid-Base & Electron Energy- 8 General Approach: 1. Look at all possible conjugate bases. (WRITE STRUCTURES!!!!) 2. Predict the relative energies of the bases. 3. Since the acid for all of the bases is the same compound, the lowest energy base will predominate and the proton whose loss produces that base will be the most acidic proton in the molecule. a. Look at all possible conjugate bases. (WRITE STRUCTURES!!!!) H H H H H H H C H b H a. H Hb H H c H C C C H cH a c H H C H H C C H C H H C H C C H H H a. HEE's on a Negative sp Carbon C H H C H C H H H 2 c. HEE's on Negative sp Carbon H H C C H C C H C C H H H C H d. Negative HEE's delocalized over a 3 Carbon atom pi system H H C b H H H H a H H H H C H H H H C C Hd C C C C H H C d H H H C C H H H C C C H H H b. HEE's on Negative sp3 Carbon C All protons in the conjugate acid are attached to carbon atoms but they differ in the hybridizations that describe their structures. There are 4 possible types of conjugate bases as indicated above (a.->d.). All have the same ionic charge and the same nuclear charge. In (a.) the HEE's are isolated in an sp hybrid orbital on a single negative carbon atom. In (b.) the HEE's are isolated in an sp3 hybrid orbital on a single negative carbon atom. In (c.), the HEE's are isolated in an sp2 hybrid orbital on a single negative carbon atom. In (d.), the HEE's are delocalized over a 3 carbon atom system. From our experience with hybridization and delocalization effects on electron energies we learned that the high effective nuclear charge available to electrons in a sp orbital (such as in conjugate base a.) lowers the energy of its electrons to a greater extent than do sp2 (c.) or sp3 (b.) orbitals or delocalization over carbon systems such as in d. The most acidic proton is the one that produces the lowest energy conjugate base when it is lost. SO: PROTONS (a) ARE MOST ACIDIC Acid-Base & Electron Energy- 8 3 b. Look at all possible conjugate bases. (WRITE STRUCTURES!!!!) O (C) CH3 a C CH2 b H c O O CH3 O C CH2 CH3 C O CH2 H The lone pair of e-'s is delocalized over two oxygen atoms due to resonance. This is the LOWEST ENERGY BASE O (B) O O (A) CH3 C CH O CH2 C CH2 H O Isolated lone pair of e-'s on sp3 carbon. This is the highest energy base. H CH3 O C CH The lone pair of e-'s is delocalized over a carbon and an oxygen as indicated by the resonance structures. Intermediate energy base. Base C has the lowest energy PROTON c IS MOST ACIDIC O 4 Acid-Base & Electron Energy- 8 c. Look at all possible conjugate bases. (WRITE STRUCTURES!!!!) H c H a H2N b H H H H H H (D) H (C) H H2N H HN (B) OH H H H OH H H - H H H2N - H OH H H H H H H2N H OH H - H - H E: Isolated lone pair of e-'s on oxygen. Lowest energy base. Effective Nuclear Charge Effect of the oxygen atom. H H2N d (A) H H (E) c H O OH H - H2N H e H H D: The lone pair of e-'s is delocalized, as OH indicated by the resonance structures, over 5 carbon atoms. This yields a small energy H decrease as in (B). Second highest energy base. Lowest Effective Nuclear Charge, small Delocalization Effect and Moderate Inductive Effect of the adjacent oxygen atom. H - C: Isolated lone pair of e-'s on an sp2 carbon atom. Intermediate energy. Lowest Effective NuclearCharge and a Weak Hybridization Effect. H H H H H H Isolated lone pair of e-'s H N - OH 2 H2N BASE (E) IS LOWEST ENERGY OH H2N OH on nitrogen PROTON e IS MOST ACIDIC Second lowest energy H H -H H H base. Intermediate H H H H Effective Nuclear B: The lone pair of e 's is delocalized, as indicated by resonance structures, over 5 carbon atoms. This yields a Charge Effect of small stabilization as in (D). Highest energy base. Lowest Effective Nuclear Charge, small Delocalization the nitrogen atom. Effect and small Inductive Effect of the adjacent nitrogen atom. Acid-Base & Electron Energy- 8 5 d. Look at all possible conjugate bases. (WRITE STRUCTURES!!!!) b H H H + H2N H H H NH3 + H2N - H H + NH3 H H NH3 H H H - + HN B: The newly generated lone pair of e-'s is on a negatively charged on sp2 carbon. Highest Energy Base. NH3 - H HN H H -HN H C: Neutral molecule. The newly generated lone pair of e-'s is on a neutral nitrogen atom. Lowest Energy Base. (B) + H NH2 H b (A) H2N c H H (C) NH3 a H Base C has lowest energy PROTON c IS MOST ACIDIC H A: The newly generated lone pair of e-'s is delocalized over a nitrogen atom and the ring. However, there is an excess of e- density causing the negative charge and increased e-- erepulsion. Intermediate Energy 6 Acid-Base & Electron Energy- 8 3. For each of the following acid-base reactions use the change in energy of the highest energy e-'s to predict whether the equilibrium constant should be > or < 1. Explain the logic that led you to your conclusion being sure to identify the highest energy e-'s of the reactants and products. a. O C OH + O O C O - + OH Here again we have competing effects. The net reaction results in the movement of the highest energy lone pair of e-'s from one delocalized system with a negative charge and an oxygen to another delocalized system with a negative charge and two oxygens. O O O C O O Reactant HEE's C O Product HEE's So the difference here is between the energy decrease of an e- pair by delocalization into a phenyl ring vs. a shorter system with 2 oxygen atoms. Since we know that the higher effective nuclear charge of one oxygen is more favorable (pKaH ~5 for carboxylate ion) than extending the delocalized system over the carbon atom of a benzene ring (pKaH ~10 for phenolate ion), the carboxylate delocalization is more effective than phenyl delocalization in lowering the energy of e- pairs. So the products of the reaction should have lower energy than the reactants and the reaction is favorable. The equilibrium constant is > 1. b. O C OH + O O O O O C O C O + C OH O O Here there is a movement of the highest energy e-'s between two similar carboxylate ions. The energies of both e- pairs are lowered by delocalization, but there is no difference in delocalization so it is not a key factor in this case. So the inductive effects are the determining factors here. Since oxygen has a higher ENC than carbon or hydrogen, the methoxy group exerts an electron withdrawing inductive effect on the HEE's on the negative oxygen decreasing e- - e- repulsion and lowering the energy of the HEE's in both the reactants and products. Since the methoxy oxygen atom is closer to the HEE's of the products than to those in the reactants, its inductive effect lowers the energies of the HEE's of the reactants more than it does those in the products. Consequently, the reactants have lower energy than the products. So the reaction is unfavorable. The equilibrium constant is < 1. Acid-Base & Electron Energy- 8 7 4. For the following pair of equilibrium controlled reactions use the change in energy of the highest energy e-'s to predict which reaction should produce the more products at equilibrium. Explain the logic that led you to your prediction being sure to identify the highest energy e-'s of the reactants and products of each reaction. O CH3 CH2 A O O C O CH3 CH2 CH2 C C O + CH3 O CH3 CH2 O + CH3 CH2 HEE CH3 CH2 - C O O HEE Delocalized carboxylate pKaH ~ 5 O- C O C CH3 O B + O HEE CH2 CH3 CH2 C O + CH3 CH2 Isolated on sp3 O HEE pKaH ~ 16 O O The HEE in the reactants are on the same structure, the phenolate ion, while the difference in structure is in the neutral compound. So the major effect of the structural differences on HEE's occurs in the products where the sites of structural difference hold the HEE's. The e-'s on the isolated sp3 O ion of the products of the second reaction are higher in energy than the delocalized O in the carboxylate ion of the products of the first reaction. Consequently we predict that delocalization will lower the energies of the HEE's in the first set of products more than those of the isolated sp3 O ion of the second set of products and also more than the delocalization of the phenol in the reactants. (See question 3. a. above) Since the reactants are approximately equal in energy because the sites of difference are fully bonded, the first reaction produces more products at equilibrium. 8 Acid-Base & Electron Energy- 8 D. Nomenclature of Aldehydes and Ketones References: 3. Applications a. Name the following: O O 3-hexanone O 2-ethylpentanal O 4-ethoxy-3-hexanone O 2,3-dimethyl-2-butenal b. Draw structural formulas for the following compounds: O O O O O Br 3-phenyl-2-pentanone 4-bromo-3-ethyl-5-heptenal 3-cyclopentenone 4-methoxybenzaldehyde