Java Based Distributed Learning Platform
advertisement

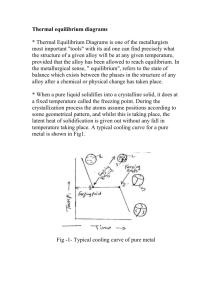
Oct. 2008, Volume 2, No.10 (Serial No.11) Journal of Materials Science and Engineering, ISSN1934-8959, USA Study on microstructure and thermal parameters of CuTeSeFe alloy ZHANG Rui-jun1, REN Yan-jun2, HE Miao1, LIU Jian-hua1 (1. State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China; 2. Analysis and Measure Center, Hebei Normal University of Science & Technology, Qinhuangdao 066004, China) Abstract: Microstructure of CuTeSeFe alloy was studied by means of metallograph, SEM/EDS and XRD, and the thermal diffusivity coefficient, thermal capacity and thermal conductivity of CuTeSeFe alloy under 25-400℃ were measured by TC-7000 thermal constant equipment. The results showed that the CuTeSeFe alloy was composed of α(Cu) solid solution and CuSeTe intermetallic compound appeared as irregular particles, and with increasing heating temperature, the thermal diffusivity coefficient decreased, the thermal capacity and thermal conductivity showed fluctuation with a small fluctuation margin. Key words: CuTeSeFe alloy; microstructure; thermal parameters; temperature 1. Introduction Copper alloy has many advantages, such as high strength, good electric conductivity and thermal conductivity, so it has been widely used in fields of electrode and electricity contact material etc. In recent years, with the large scale integration and micromation of electron circuitry, there are higher thermal conductivity requirements to materials, and the heat dissipation of conventional materials is not enough, especially working at high temperature. Hence, the research on producing copper alloy with high strength and good thermal conductivity attracted many researchers’ attention[1-4]. Some researches showed that the addition of some microelement into copper alloy was one of effective method to improve their comprehensive properties[5-7]. Therefore, in this paper, we choose self-made CuTeSeFe alloy as experimental material and measure its thermal parameter in the temperature range of 25-400℃, it provided fixed reference data for the application of the copper alloy. 2. Experimental The material is annealed CuTeSeFe alloy, with the chemical composition (mass fraction, %) of 96.70Cu, 1.62Se, 1.09Te, 0.35Fe and 0.24 others. The material was melted in the vacuum medium frequency induction furnace and then cast to bar, and the bar was machined to Φ10mm×1.75 mm. After grinding by 1200# sand paper and then the thermal diffusion coefficient(K), heat capacity(C) and thermal conductivity(λ) were continuous measured at 25℃, 100℃, 200℃, 300℃ and 400℃, respectively. The microstructure were observed and analyzed by Neophot21 type optical microscope, KYKY-2800 type scanning electron microscope and D/MAX-rB type X-ray diffraction (with graphite monochramator, Kα radiation). 3. Results and discussion 3.1 Microstructure Fig. 1 shows the microstructure of the CuTeSeFe alloy. It can be seen that as-cast microstructure consists of irregular granular and matrix, and it can be known from the XRD analysis (Fig. 2) that the CuTeSeFe alloy is mainly composed of α phase (Cu solid solution) and Cu4SeTe intermetallic compound. Corresponding author: ZHANG Rui-jun (1962- ), male, Ph.D., professor; research field: metallic materials. E-mail: Zhangrj@ysu.edu.cn. 31 Study on microstructure and thermal parameters of CuTeSeFe alloy microstructure of the CuTeSeFe alloy after treatment at 25-400℃ and that before treatment, and the XRD analysis indicate that there is no new phase formed in the CuTeSeFe alloy after treatment at 25-400℃. It means that during heating process at 25-400℃, the microstructure of the CuTeSeFe alloy has little change. Cu Fig. 1 Microstructures of original CuTeSeFe alloy 20000 ● ●——α(Cu) 15000 Intensity /counts 2500 (a) matrix 2000 1500 Cu 1000 ▲——Cu4SeTe Fe Cu CPS 500 10000 ● ● Fe Fe 0 0 5000 ● Cu Fe keV 5 10 15 Energy/keV ● ▲ 0 Cu Cu 600 40 60 80 100 (b) granular 2θ/(º) Fig. 2 X-ray diffraction pattern of original CuTeSeFe alloy EDS analysis (Fig. 3) shows that the matrix is mainly consist of Cu and Fe, and irregular granular is compose of Cu, Se and Te (Table 1). It can be concluded that the matrix is α phase, and irregular granular is CuSeTe intermetallic compound (Such as Cu4SeTe). From metallographic analysis, it can be also observed that there is no difference between the 500 Intensity /counts -5000 20 400 300 Se Te 200 Cu Se 100 Se Te Cu Se 0 0 5 10 keV 15 Energy/keV Fig. 3 EDS spectra of the CuTeSeFe alloy Table 1 Data of EDS analysis (mass fraction, %) Phase Matrix Granular Cu 99.51-99.65 65.23-73.05 Fe 0.27-0.34 / 3.2 Thermal parameters Fig. 4 shows the effect of heating temperature on the thermal diffusivity coefficient of the CuTeSeFe alloy. It can be seen that the thermal diffusivity coefficient of the CuTeSeFe alloy decreases with increasing temperature, and when temperature is increased from 25℃ to 400℃, the thermal diffusivity 32 Se / 14.18-17.72 Te / 12.69-14.97 Others 0.16-0.21 / coefficient is decreased from 0.8148 cm2.sec-1 to 0.6846 cm2.sec-1. Furthermore, the thermal diffusivity coefficient at temperature from 25℃ to 200℃ decreases more greatly than that at temperature from 200℃ to 400℃. According to experimental data, equation between the thermal diffusivity coefficient Study on microstructure and thermal parameters of CuTeSeFe alloy 2.0 (K) and temperature (t) were gained as follows by unitary linearity regression. 10 ●—λ 1.6 8 0.84 K/cm2.sec-1 0.80 0.76 0.72 6 0.8 4 0.4 2 0.0 0.68 0.64 1.2 0 90 180 270 360 λ/W.cm-1.k-1 C/J.g-1.k-1 ■—C 0 450 T/℃ 0 100 200 300 400 500 Fig. 5 Relationship between the thermal capacity or thermal conductivityof CuTeSeFe alloy and heating temperature T/℃ Fig. 4 Relationship between the thermal diffusivity coefficient of CuTeSeFe alloy and heating temperature K =-0.0005t+0.8298, t: 25-200℃; K =-0.0002t+0.7641, t: 200-400℃. Fig. 5 shows the relationship of the thermal capacity or thermal conductivity of CuTeSeFe alloy versus heating temperature. From Fig. 5, it can be found that the thermal capacity and thermal conductivity of the CuTeSeFe alloy show fluctuation with a small fluctuation margin at 25-400℃, the thermal conductivity and heat capacity varies from 0.7747 J.g-1.k- to 0.8899 J.g-1.k-1 and from 5.497 W.cm-1.k-1 to 5.898 W.cm-1.k-1 respectively in the temperature range of 25-400℃. As a result, when temperatures are 300℃ and 100℃, the highest value and the lowest value of thermal capacity of the CuTeSeFe alloy are 0.8899 g-1.k-1 and 0.7747 g-1.k-1, respectively, and a difference of about 14.87 % in thermal capacity between the highest value and the lowest value. For the thermal conductivity of the CuTeSeFe alloy, when temperatures are 25℃ and 200℃, the highest value and the lowest value of thermal conductivity are 5.898 W.cm-1.k-1 and 5.497 W.cm-1.k-1, respectively, and a difference of about 6.79 % in thermal conductivity between the highest value and the lowest value, 3.3 Discussion In general, in the process of the heat transfer, free electron and phonon knock atom and molecule. Meanwhile, the scattering of free electrons and phonons are aggravated caused by interface and all kinds of defects, resulting in thermal resistance forms[8]. Therefore, the more integrated the metal crystal is, the less of the defects of crystal distortion and grain boundary caused by inhomogeneous atom, the more easily electron passes, the better the thermal conductivity of the metal. With the increase of the heating temperature, on the one hand, the movement of the atoms was speed up, the number of the collision between the atoms was increased, heat vibrancy of the crystal lattice was speed up, and the crystal defect was manifold. In that case, the dispersion to the moving electrons will be increased, the movement of the free electron in the matrix will be restrained, and the heat diffused coefficient of the atoms will be decreased, thus, the thermal conductivity of the alloy was decreased, while on the other hand, due to the increase of the heating temperature, the internal stress in the as-cast sample was cleared up, it is to say, that the thermal wasting was eliminated[9], resulting in the thermal conductivity of the alloy increases. Summing up the function of the two above-mentioned aspects, the thermal conductivity of the alloy may be fluctuated with the increase of the temperature. 33 Study on microstructure and thermal parameters of CuTeSeFe alloy The conduction of the CuTeSeFe alloy consisted of the conduction of Cu matrix and irregular granular intermetallic compound, the conduction of Cu matrix was mainly depended on the free electron, and the conduction of irregular granular intermetallic compound was mainly depended on the free phonon[10]. Because Se and Te almost didn’t solid solution with Cu, only formed a compound with higher melting point, it could be confirmed that Se and Te didn’t affect the microstructure of the CuTeSeFe alloy during the heating in the experimental temperature range. Thus, Se and Te affected very little to the thermal conductivity of the alloy during the experiment process, and the conduction of the Cu matrix played a main role in the thermal conductivity change of the CuTeSeFe alloy. 4. Conclusions (1) The microstructure of the CuTeSeFe alloy is consisted of α(Cu) solid solution and irregular granular CuSe(Te) intermetallic compound. (2) The thermal diffusion of the CuTeSeFe alloy decreases with increasing heating temperature, in the temperature ranges of 25-200℃ and 200-400℃, and the relationship between thermal diffusion (K) and temperature (t) can be explained as K=-0.0005t+0.8298 and K=-0.0002t+0.7641, respectively, The thermal capacity and thermal conductivity of the CuTeSeFe alloy show fluctuation with a small fluctuation margin at 25-400℃. References: [1] D.R. Poirier, E. McBride. Thermal conductivities of hypoeutectic AI-Cu alloys during solidification and cooling. Materials Science and Engineering, 1997, A224: 48-52. 34 [2] WANG Xiao-liang, LIU Yi, LIU Wei-hua. Study on sintering process for preparing Cu-Al gradient function material. Hot Working Technology, 2007, 36(10): 57-59. [3] S. Suzuki, K. Hirabayashi, H. Shibata. Electrical and thermal conductivities in quenched and aged high-purity Cu–Ti alloys. Scripta Materialia, 2003, 48: 431-435. [4] R. Zengin, F. Yakuphanoglu. Effects of hydrostatic pressure on the martensitic phase transformation, thermal conduction and thermodynamic parameters of Cu-13 wt.% Al-4 wt.% Ni shape memory alloy. Materials Letters, 2003, 57: 3107-3110. [5] MU S. G., GUO F. A., TANG Y. Q.. Study on microstructure and properties of aged Cu-Cr-Zr-Mg-Re alloy. Materials Science and Engineering: A, 2008, 475(1-2): 235-240. [6] GAO L. L., CHENG X. H.. Microstructure, phase transformation and wear behavior of Cu-10%Al-4%Fe alloy processed by ECAE. Materials Science and Engineering: A, 2008, 473(1-2): 259-265. [7] CAI Wei, LIU Rui-qing, WANG Xiao-juan. Effects of contents of Zr and Cr on structure and property of copper alloy. Hot Working Technology, 2006, 35(6): 10-11. [8] TAO Ning, FENG Yi, WANG Cheng-fu. The effect of distribution of fibers on the thermal conductivity of carbon fiber-copper composites. Materials Review, 1999, 13(6): 60-64. [9] CHEN De-xin, WANG Zhi-fa, JIANG Guo-sheng. Effect of residual stresses on the thermal conductivity of W-Cu composites. Rare metal Materials and Engineering, 2005, 34(12): 1982-1984. [10] ZHANG Qiang, CHEN Guo-qin, JIANG Long-tao. Thermal conduction properties of aluminum matrix composites reinforced withdual-sized particles. Acta Materiae Compositae Sinica, 2005, 22(1): 47-51. (Edited by Tsyung and Emily)