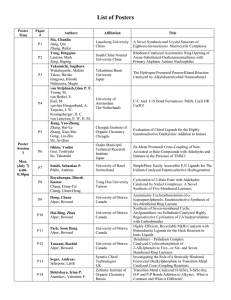
Abstracts of Plenary Presentations, Short Oral Communication, and
advertisement

Solid-Supported Reagents in the Synthesis of Lamellarins and Deprotection of Aromatic Ethers Poonsakdi Ploypradith,* Kassrin Tangdenpaisal, Supannee Sualek, Rachel K. Kagan, Daniel R. Bertoni, Vanessa Guzman, Somsak Ruchirawat Laboratory of Medicinal Chemistry, Chulabhorn Research Institute, and Program in Chemical Biology, Chulabhorn Graduate Institute, Vipavadee-Rangsit Highway, Bangkok 10210 Thailand. E-mail: poonsakdi@cri.or.th Recently, we have reported the applications of solid-supported reagents in the synthesis of lamellarins, a group of marine natural products derived from sponges and ascidians. Three solid-supported reagents were employed successively in three reactions of keto alphabromination, base-mediated condensation to assemble the pyrrole core, and acid-mediated transacylation followed by lactonization. Bromine on solid supports (Amberlyst A-26 Br3- and polymer bound pyridine hydrobromide perbromide (PVPHP)) furnished the desired phenacyl bromide carbonate derivatives in good yields with remarkable selectivity (the ratio of mono:di up to 35:1). Amberlyst A-26 NaCO3was effective for the condensation reactions of benzyldihydro-isoquinoline with the derivatives of phenacyl bromide or alpha-nitrocinnamate, X X R 1O O R 1O N R 2O R 3O A m b e r ly s t A - 2 6 N a C O 3 R 5O C O 2E t R 6O R 4O O Bn A m b e r ly s t A - 2 6 N a C O 3 O R 5O Br R 6O NO2 O C O 2E t R 4O R O C O 2E t OBn OR6 OR5 A m b e r ly s t- 1 5 R 1O A m b e r ly s t A - 2 6 B r 3 - R 5 O R 6O O C O 2E t M eO CHO or P V P HP O R 4O OR6 OR5 R 4O O M eO RO O OR6 OR5 A m b e r ly s t- 1 5 R 1O M eO X Y R = M e o r i- P r ; R 1 = i- P r ; X = H , O B n , o r O i- P r ; Y = H or O M e O C O 2E t OMe R = M e o r i- P r OR O C O 2E t Br R 6O i- P r O RO R = i- P r , tert- B u , a lly l, Bn, P M B, M O M 2 ,3 - d im e th o x y b e n z y l, 2 ,3 ,4 - tr im e th o x y b e n z y l O N O R 5O X R 2O R 3O X N N R 2O R 3O - 1 R 2O R 3O forming the pyrrole core. The Amberlyst-15mediated reactions of the 2H-pyrrole or the Obenzyloxy pyrrole completed the synthesis. In addition, p-TsOH immobilized on either silica (PTS-Si) or polystyrene (PS)—divinyl benzene (DVB) polymer (Amberlyst-15) was general and effective for the deprotection of aromatic ethers. The i-Pr, tert-Bu, Bn, PMB, allyl, MOM, di- and tri-methoxylated benzyl protecting groups could be removed. When these ethers were simultaneously present in the same molecule but on different aromatic rings, or even on the same aromatic ring, moderate to excellent selectivity of their removal could be obtained. The corresponding carbocations generated from these protecting groups were proposed as important intermediates. O M eO NO2 OMe R 1O OR2 R 1 = R 2 = B n , i- P r , a lly l, o r P M B Ploypradith, P.; Cheryklin, P.; Niyomtham, N.; Bertoni, D. R.; Ruchirawat, S. Org. Lett. 2007, 9, 26372640. Petchmanee, T.; Ploypradith, P.; Ruchirawat, S. J. Org. Chem. 2006, 71, 2892-2895. Ploypradith, P.; Kagan, R. K.; Ruchirawat, S. J. Org. Chem. 2005, 70, 5119-5125. Poonsakdi Ploypradith (พูนศักดิ์ พลอยประดิษฐ์ ), b 1971 in Bangkok, Thailand. The Johns Hopkins University (BA 1994), The Johns Hopkins Univ. (PhD 1999, Prof. G. H. Posner), Research Scientist, Chulabhorn Research Institute (2000-present), Lecturer, Chulabhorn Graduate Institute (2007-present). Research field: total synthesis of natural products and synthetic methodology.