Protein-Protein Interactions in Membrane Traffic Elucidated by
advertisement

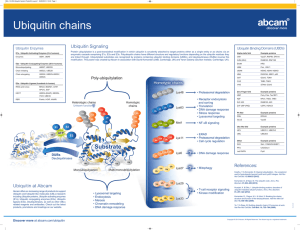
PROTEIN-PROTEIN INTERACTIONS IN MEMBRANE TRAFFIC ELUCIDATED BY SYNCHROTRON X-RAY PROTEIN CRYSTALLOGRAPHY Soichi Wakatsuki Structural Biology Research Center, Photon Factory, IMSS, KEK, Tsukuba, Ibaraki, Japan. Membrane traffic plays crucial roles in cell functions such as post-translational modification of newly synthesized proteins, exocytosis and endocytosis, receptor recycling and autophagy. Vesicle transport mediates these trafficking events using an intricate network of protein-protein interactions of coat proteins, adaptor proteins (AP), cargo receptors, SNARE complexes, small GTPases, ubiquitin and various accessory proteins. Synchrotron X-ray protein crystallography has been instrumental in elucidating their key interactions at the atomic level. In this talk, I will start with a short presentation on high throughput technologies for synchrotron-based structural genomics followed by description of our recent studies on membrane traffic between the trans Golgi Network and endosomes/lysosomes, and ubiquitin recognition in the receptor recycling. GGAs (Golgi-localizing, -adaptin ear homology domain, ARF-binding proteins) are important for clathrin-coated vesicle transport between the TGN and endosomes/lysosomes, classical AP-1 complexes (Fig. 1, see review [1]). They consist of three domains and a hinge region, each of which interacts with other transport proteins. The N-terminal VHS domain binds TGN sorting receptors, such as mannose 6-phosphate receptors [2], sortilin and -secretase [3] by recognizing their acidic cluster dileucine sequences. The GAT domain uses its hydrophobic residues to dock onto a small GTPase, ARF thereby tethering GGA to the TGN membrane for efficient recruitment of cargo receptors by the VHS domain [4]. Its C-terminal half interacts with ubiquitin [5] and Rabaptin-5, thus modulating the GGA’s function. The proline-rich hinge region has a variety of binding partners: the N-terminal -propeller of clathrin, the VHS and GAE domains. The -ear domain of AP-1 [6] and the GAE domain adopt very similar immunoglobulin folds to interact with Rabaptin-5 and -synergin and other accessory proteins. More recent examples of ubiquitin recognition by Hrs, GGA, and Tom1 will be presented to illustrate the recurring structural theme, dual binding of ubiquitin molecules in recycling of receptors. Figure 1. Interaction partners of GGA proteins References [1] - K. Nakayama & S. Wakatsuki, Cell Struct. Funct. 28, 431, 2003 [2] - T. Shiba et al. Nature 415, 937, 2002 [3] - T. Shiba et al. Traffic 5, 437, 2004 [4] - T. Shiba et al. Nature Structural Biology 10, 386, 2003 [5] - Y. Shiba et al., J. Biol. Chem. 279, 7105, 2004 [6] - T. Nogi et al. Nature Structural Biology, 9, 527, 2002