Laboratory Assistance Visit Form
advertisement

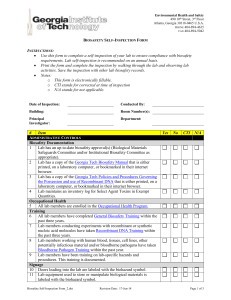
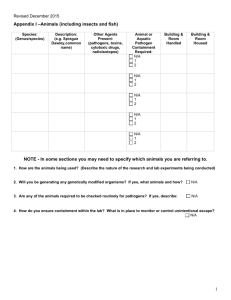
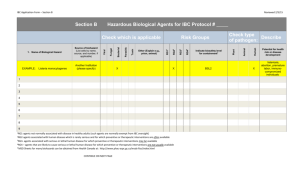
Version: 11/2011 Georgia Regents University Institutional Biosafety Committee (IBC) – Laboratory Assistance Visit Checklist A procedure-based approach comprising the practices, personal protective equipment, facilities and implementation of your laboratory’s Standard Operating Procedures (SOPs) must be taken when evaluating safety and compliance. This checklist is recommended for use as a tool for self-assessment and is used to review of your laboratory safety by the Biosafety Office as required by the Institutional Biosafety Committee. Principle Investigator/Representative: Date: Assistance Visit Performed by: Location(s): All standards described below are based on those from the following publications: GHSU Institutional Biosafety Guide (BSG) http://www.georgiahealth.edu/services/ehs/biosafe/PDFs/bioguidejun08.pdf CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th edition: NIH Guidelines for Research Involving with Recombinant DNA Molecules: OSHA Bloodborne Pathogen (BBP) Standards http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm http://oba.od.nih.gov/rdna/nih_guidelines_oba.html http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051 A. Work Practices (Standard Microbiological Practices) 1 2 3 4 5 6 7 8 Is access to the laboratory limited to laboratory staff when experiments/work with biological materials or recombinant DNA are in progress? Does laboratory staff wash hands after handling viable materials, after removing gloves, and before leaving laboratory? Are supplies/materials available in each laboratory area to facilitate this operation (soap, paper towels, and clear sinks)? Are gloves disposed of immediately prior to washing hands? Are eating, drinking, smoking, handling contact lenses, or applying cosmetics, forbidden in the laboratory? Is disposal of food/drink wrappers and containers forbidden in the laboratory? Are utensils (cups, plates, forks, etc.) used in consuming food forbidden in the laboratory? Is food for human use stored outside of the laboratory work area in refrigerators designated for this purpose only? Is a floor-to-ceiling/wall-to-wall barrier between laboratory areas and non-laboratory areas (offices, hallways, etc.) in which food is stored/consumed? Are only mechanical pipetting devices used (e.g., is mouth pipetting forbidden)? Are sharps safety devices employed whenever possible to avoid sharps injuries? Have sharps safety device options been considered and documented? Are sharps NOT bent, sheared, broken, recapped, removed from disposable syringes, or manipulated by hand? Response Comments Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A 1 9 10 11 12 13 14 15 16 17 Are sharps placed in red (approved) sharps containers? Are sharps containers placed near the areas where sharps are used to facilitate immediate disposal? Are sharps (razors, needles, scalpels) NOT left unprotected and/or exposed on bench tops or shelves; which would permit inadvertent sharps injury? Non-disposable sharps must be placed in a hardwalled container for storage and transport. Are sharps containers disposed of periodically and not allowed to overfill? Containers should be closed upon reaching the fill line (approximately 2/3 full) and disposed of immediately or at least every six months, as per GHSU procedures. Are all biologically contaminated solid wastes decontaminated as per the laboratory’s IBC-approved SOPs and disposed of as biomedical waste? Are appropriate disinfectants easily available within the laboratory? Are disinfectants prepared and used according to IBC-approved SOPs or manufacturer’s standards? Are sufficient supplies and equipment available to accomplish this disinfection? Are biohazard boxes easily available within the laboratory? Are biohazard boxes disposed of promptly and not allowed to overfill? Is laboratory staff familiar with the solid waste disposal procedures documented in your SOPs? Are all biologically contaminated liquids/fluids decontaminated and disposed as indicated in the laboratory’s IBC-approved SOPs? Are appropriate disinfectants easily available within the laboratory? Are there appropriate collection containers for safe collection of liquid wastes with secondary containment? Are the liquid wastes decontaminated and disposed on a regular basis (e.g., not allowed to sit for lengthy periods and not allowed to fill the container over ½ full)? Are laboratory staff familiar with the liquid waste disposal procedures documented in your SOPs? Are vacuum lines protected by in-line HEPA filters? Are aspirator traps set up correctly (in secondary containment and not permitting waste to build up into the neck of the flask)? Are work surfaces decontaminated with disinfectants that are effective against the agents of concern at the completion of work or at the end of the day and after any spill or splash of viable material? Are appropriate disinfectants easily available within the laboratory? Are sufficient supplies and equipment available to accomplish this disinfection? Are disinfectants prepared and used according to IBC-approved SOPs or manufacturer’s standards? Are protective coverings (plastic wrap, aluminum foil, imperviously-backed absorbent paper) removed or replaced when contaminated and/or at the end of the work shift? Is laboratory staff familiar with the work surface decontamination procedures documented in your SOPs? Is equipment decontaminated with disinfectants that are effective against the agents of concern on a regular basis, before being removed from the laboratory, before repair/contact of unauthorized personnel and after spills/splashes? Is laboratory staff familiar with the equipment decontamination procedures documented in your SOPs? Is equipment in which biological materials are stored labeled with a biohazard sticker (e.g., freezer, refrigerators, cryotanks, etc.)? Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A 2 18 19 20 21 22 23 Are all animal carcasses disposed in the animal carcass freezers designated by LAS facilities and NOT in any biohazard waste container? If research involves animals requiring ABSL-2 containment, are the carcasses disposed within the ABSL-2 facility? If any hazardous drugs, chemicals, chemotherapeutic agents or radiological materials have been introduced into the animals, are the carcasses appropriately segregated and disposed of according to the laboratory’s IBC-approved SOPs? If the laboratory procedures involve the transport of animals, are the animals transported using filter-top cages, with the top secured by positive means (e.g, rubber band, tape), and the caged draped (e.g.,if transporting multiple cages, are cages placed on the lower shelf of a cart)? If produced, are the mixed wastes (biological + chemical and/or biological + radiological) appropriately decontaminated for biological agents before being disposed as per the laboratory’s IBC-approved SOPs through the Chemical Safety or Radiation Safety Offices? If the laboratory procedures involve intramural transport of biological materials outside of the laboratory, does the laboratory have the appropriate containment device(s) available (e.g., a sealed, leakproof primary container inside a well-labled, sealed, leakproof durable secondary container)?. If the laboratory procedures involve shipping, extramural transport, importation/exportation of biological materials or items on dry ice/liquid nitrogen, does the Biosafety Office have copies of current IATA (Saf-T-Pak) training certificates for all designated shippers? Are appropriate packaging materials available that comply with the IATA standards for the agents being shipped? Is a Biohazard placard posted at entrance(s) to laboratory? Is the primary and secondary contact information current? Are the lists of agents current? Are the precautionary measures (PPE, vaccinations) listed current? B. Special Work Practices 24 25 26 27 28 29 Has the PI established policies and procedures whereby only persons who have been advised of the potential hazards and met specific entry requirements (e.g. immunization) are allowed to work biological materials or recombinant DNA? Does the PI ensure that laboratory staff are screened by Occupational Health and provided the appropriate immunizations, response plans, etc., as required by the IBC for the hazard to which they have a reasonable expectation for exposure? Does the PI ensure that persons who are at an increased risk of acquiring infection or for whom infection may have serious consequences are not allowed to enter the laboratory when work with infectious agents is in progress without permission of the PI? Has the PI developed a laboratory-specific IBC-approved Biosafety Protocol and SOPs? Have these documents been discussed and made available in the laboratory for reference? Has the PI ensured that the laboratory staff have been trained and demonstrated proficiency in implementing the laboratory’s IBC-approved SOPs? Has this laboratory-specific training been documented? Are spills, accidents, and/or injuries that result in overt exposures to biological materials immediately reported to the PI? Does the PI report these to the Biosafety Office as required by the IBC? Is appropriate medical evaluation provided? Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes Yes Yes No No No N/A N/A N/A Response Comments Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A 3 30 31 32 Are laboratory staff aware of the spill, exposure, accident, injury procedures documented in the laboratory’s IBC-approved SOP (e.g., whom to contact, and the location of health care providers)? Are animals and plants not associated with the work being performed forbidden into the laboratory? If toxins are being used, are special practices, equipment and storage being utilized as per the laboratory’s IBCapproved SOPs? If Select Agents and Toxins (SATs) are being used, are special practices, equipment and storage being utilized as per the laboratory’s IBC-approved SOPs? C. Safety Equipment (Primary Barriers and Personal Protective Equipment) 33 34 35 36 37 38 39 40 41 Does the laboratory have a Biosafety Cabinet or other physical containment device for procedures with a potential for creating infectious aerosols or splashes, or whenever handling high concentrations of infectious materials? Has the Biosafety Cabinet been certified annually or after installation, relocation or repair? Is the Biosafety Cabinet installed away from doors, high traffic areas, ventilation devices, or other equipment that could disrupt airflow patterns needed for containment? Is laboratory staff trained to use the Biosafety Cabinet appropriately? Are the grates in the front and back of the Biosafety Cabinet unblocked? Are drop trays disinfected routinely or after spills by laboratory staff? Are rapid in/out and side-to-side arm motions avoided? Is open flame, volatile chemical or radioactive material use avoided in the Biosafety Cabinet, unless special equipment and procedures have been detailed in your IBC-approved SOPs? Does the centrifuge(s) used for potentially infectious agents have sealed rotor heads or centrifuge safety caps? Are safety caps opened only in a Biosafety Cabinet? Is protective clothing removed and left in the laboratory before going to non-laboratory areas (cafeteria, library, administrative areas)? Is protective clothing either disposed of in the laboratory or laundered by institution? (NEVER taken home!) Is the procedure for laundering/decontaminating protective clothing understood by the laboratory staff? Is face protection (goggles, mask, face shield, or other splatter guards) used as per the laboratory’s IBC-approved SOPs? Are sufficient supplies available? Are gloves changed frequently, when contaminated or torn (NEVER reused)? D. BSL -2 Laboratory Facilities (Secondary Barriers) 42 43 44 45 46 47 Does the laboratory have doors that lock? Is the laboratory designed so that it can be easily cleaned and is the laboratory maintained clean? (Carpets and rugs are not allowed in laboratory work areas and all furniture must be non-porous; no cloth chairs, curtains, wall covers, or bulletin boards). Are efforts made to easily reduce clutter of items that cannot be easily decontaminated within the laboratory? Are bench tops impervious to water and resistant to heat, organic solvents, acids, alkalis, and chemicals used to decontaminate the work surface and equipment? Is laboratory furniture capable of supporting loads and uses? Are spaces between benches, cabinets, and equipment accessible for cleaning (e.g., does the laboratory refrain from storing cardboard boxes on the floor and in cold rooms)? Are eyewash/eyewash stations readily available? Yes No N/A Yes No N/A Yes No N/A Yes No N/A Response Comments Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Response Comments Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A Yes No N/A 4 48 49 Is laboratory staff trained to locate the eye wash station with eyes closed within 10 seconds of every work area? Are the eye wash stations flushed at least once every six months by the laboratory staff? Is illumination level adequate? Is the laboratory under negative air pressure relative to the corridor or neighboring laboratory areas of lower biological risk? Yes No N/A Yes No N/A Yes No N/A Yes No N/A IMPORTANT INFORMATION: Environmental Services Manager for your building: _______________________________ (contact for disposal/supply of sharps containers and biohazard boxes) Occupational Health Office (ext. 1-3418) Laboratory Equipment Services (Biosafety Cabinet Certifier) (ext. 1-16124) ADDITIONAL COMMENTS: _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ The Principal Investigator is responsible for full compliance with the policies, practices and procedures set forth by the CDC, NIH, OSHA, and GHSU (see standard references). The PI is responsible for assuring and documenting the appropriate training of employees and for correcting errors and unsafe working conditions. Principal Investigator/Representative Signature Date Biosafety Office Representative Signature Date 5