Animals
advertisement
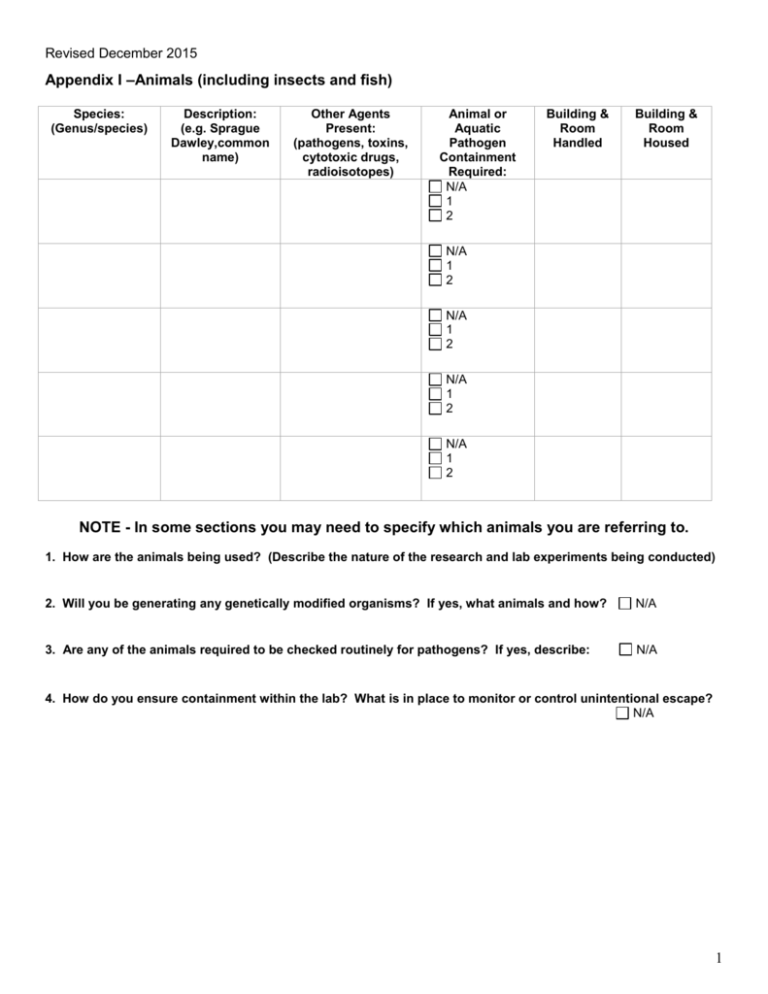
Revised December 2015 Appendix I –Animals (including insects and fish) Species: (Genus/species) Description: (e.g. Sprague Dawley,common name) Other Agents Present: (pathogens, toxins, cytotoxic drugs, radioisotopes) Animal or Aquatic Pathogen Containment Required: N/A 1 2 Building & Room Handled Building & Room Housed N/A 1 2 N/A 1 2 N/A 1 2 N/A 1 2 NOTE - In some sections you may need to specify which animals you are referring to. 1. How are the animals being used? (Describe the nature of the research and lab experiments being conducted) 2. Will you be generating any genetically modified organisms? If yes, what animals and how? N/A 3. Are any of the animals required to be checked routinely for pathogens? If yes, describe: N/A 4. How do you ensure containment within the lab? What is in place to monitor or control unintentional escape? N/A 1 Revised December 2015 Appendix I - Animals 5. Please describe how the animals in Appendix I are transported (e.g. by car, between labs/buildings) and describe the safety/containment measures taken: Attached standard operation procedures (SOPs) and/or refer to specific Vivaria and/or other biosafety guidelines that are followed. 6. Please describe how animals are handled and precautions taken for different routes of exposure: Attached SOPs, or list specific Vivaria and/or other biosafety guidelines that are followed. General Handling Procedures and Precautions: Specific Lab Precautions (based on what is being handled and what experiment is being conducted): Exposure Route/Hazard:(example) Type of activity being performed: Protective Measures: Inhalation (e.g. animal allergens) Inhalation (e.g. aerosol generation) Injection, Inoculation/Needle use, Bites/Scratches Direct and Indirect Contact 7. Regulatory requirements followed in the lab (only select what is applicable): Containment Level 1 Lab guidelines All Human Pathogens and Toxins and Terrestrial Animal Pathogen CL2* Operational Practice Requirements in Chapter 4 of the Canadian Biosafety Standards, 2nd Edition are followed, under the HPTA and HAA and pertinent regulations. All AQC** applicable requirements are met as described in the Containment Standards for Facilities Handling Aquatic Animal Pathogens. *CL2 – Containment Level 2 as defined in the Canadian Biosafety Standards, 2nd Edition. **AQC- Aquatic Containment Level (1-3) 8. Please describe the laboratory protocols followed for disinfection (e.g. lab surfaces), decontamination and disposal of biological materials and contaminated materials (e.g. PPE, paper towels, pipette tips, etc.): Attached SOPs and/or refer to specific Vivaria and/or other biosafety guidelines that are followed. 2











