week 3 inclas
advertisement
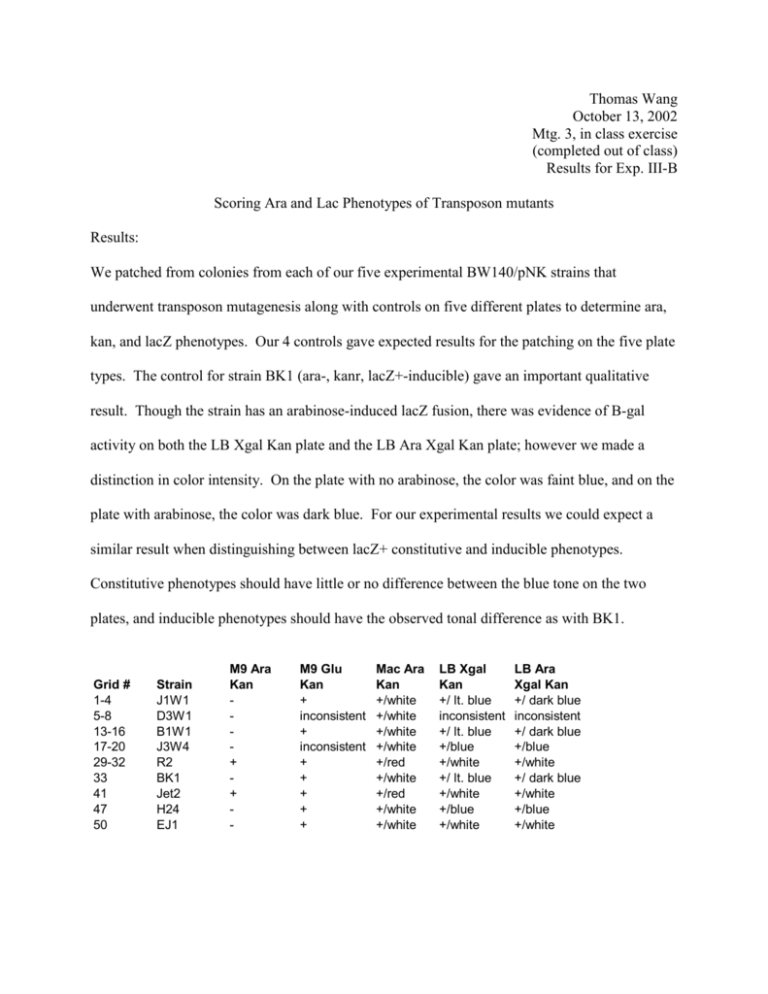
Thomas Wang October 13, 2002 Mtg. 3, in class exercise (completed out of class) Results for Exp. III-B Scoring Ara and Lac Phenotypes of Transposon mutants Results: We patched from colonies from each of our five experimental BW140/pNK strains that underwent transposon mutagenesis along with controls on five different plates to determine ara, kan, and lacZ phenotypes. Our 4 controls gave expected results for the patching on the five plate types. The control for strain BK1 (ara-, kanr, lacZ+-inducible) gave an important qualitative result. Though the strain has an arabinose-induced lacZ fusion, there was evidence of B-gal activity on both the LB Xgal Kan plate and the LB Ara Xgal Kan plate; however we made a distinction in color intensity. On the plate with no arabinose, the color was faint blue, and on the plate with arabinose, the color was dark blue. For our experimental results we could expect a similar result when distinguishing between lacZ+ constitutive and inducible phenotypes. Constitutive phenotypes should have little or no difference between the blue tone on the two plates, and inducible phenotypes should have the observed tonal difference as with BK1. Grid # 1-4 5-8 13-16 17-20 29-32 33 41 47 50 Strain J1W1 D3W1 B1W1 J3W4 R2 BK1 Jet2 H24 EJ1 M9 Ara Kan + + - M9 Glu Kan + inconsistent + inconsistent + + + + + Mac Ara Kan +/white +/white +/white +/white +/red +/white +/red +/white +/white LB Xgal Kan +/ lt. blue inconsistent +/ lt. blue +/blue +/white +/ lt. blue +/white +/blue +/white LB Ara Xgal Kan +/ dark blue inconsistent +/ dark blue +/blue +/white +/ dark blue +/white +/blue +/white We plated four ara- mutant strains and one ara+ mutant strain. R2’s phenotype is ara+, kanr, lacZ-. The R2 strain grew on both minimal media plates (M9 Ara Kan and M9 Glu Kan). This confirmed insertion of the Kanr and lacZ genes from the miniTn10 transposon in a non-Ara gene in the recipient BW140/pnK strain. This mutant also appeared red on the Mac Ara Kan plate due to its metabolism of arabinose sugar, which lowers the pH and leads to the red color. The strain appeared white on both LB Xgal plates (LB Xgal Kan and LB Ara Xgal Kan), indicating that the lacZ gene was not inserted in the correct orientation or reading frame or in an open reading frame. The kan gene is expressed regardless of the specifics of its insertion because it has its own promoter gene. We cannot conclude any phenotypic characteristics from our D3W1 and J3W4 strains. Both strains gave inconsistent results on at least one plate. This indicates that our strains were not pure since different colonies from a single strain gave different patching results. The remaining two mutant strains, J1W1 and B1W1, gave the same consistent phenotypic characteristics on all 5 plates. These two strains are both ara-, kanr, lacZ+-inducible. The strains had no growth on the M9 Ara Kan plate and growth on M9 Glu Kan plate, which confirmed kanamycin resistance and miniTn10 insertion. The strains had white colony growth on the Mac Ara Kan plate due to the ara- phenotype. These strains could not metabolize arabinose and instead metabolized amino acids, resulting in increased basicity and the white color of the colonies. Finally, both colonies had patching results on the LB Xgal Kan and LB Ara Xgal Kan plates identical to that of BK1; colonies appeared light blue on LB X-gal Kan and dark blue on LB Ara X-gal Kan plate. We concluded that these strains are LacZ+-inducible like the control BK1. We further know that miniTn10 made its insertion into either the AraA or AraB gene because these are the two inducible genes of the arabinose operon, and that this insertion occurred in the correct orientation and reading frame.