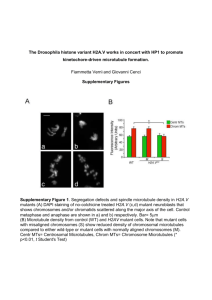
Mutant MyoD Lacking Cdc2 Phosphorylation Site Delays M
advertisement

Kim Dae-Hwan Mutant MyoD Lacking Cdc2 Phosphorylation Site Delays M-Phase Entry Lionel A. J. Tintignac et al MyoD is transcription factor in proliferating myoblast cells and it is regulated directly by both P300/CBP and P/CAF co-activators. The interaction of MyoD and HDAC1 prevents premature activation of myogenic program in growing myoblasts. Phosphorylation of MyoD at Ser 200 plays an important role in modulating its half-life and transcriptional activity during myoblast proliferation. MyoD up-regulates p21 and accumulated p21 may facilitate the integration of critical G2 checkpoint signals that regulate entry into mitosis in the G2/M-phase transition. In this paper, they show how the phosphorylation of MyoD by cyclin B-Cdc2 affects the transcription of p21 and enters into M-phase from G2 stage in the cell cycle. From MyoD accumulated at G2 stage but degraded and phosphorylated at M stage, it shows that MyoD may engage into transition from G2 to M and have a specific function G2 or M phase (Fig. 1). To identify function of MyoD, they made the mutant MyoD whose Ser 5 and Ser 200 was substituted to Ala (Fig. 2). Phosphorylation of wt-MyoD on Ser 5 and Ser 200 by cyclin BCdc2 decreases a transactivational function unlike mutant MyoD (Fig. 3.B). Phosphorylation of MyoD makes MyoD’s transcriptional activity weak and as a result, leads to its downstream genes toward down-regulation. How does MyoD regulate a transcription of downstream gene? Mutant MyoD directly interacts with P/CAF, which has acetyltransferase activity and upregulates the transcription but wt-MyoD not. On the other hand, wt-MyoD binds to HDAC1 stronger than mutant MyoD (Fig. 4). As a result, the phosphorylational state of MyoD is crucial role in activation or repression of transcription by interaction with critical effector molecular which is involved in its transcriptional activation and silencing. Mutant MyoD activates expression of p21much stronger than wt-MyoD in contrary to a cyclin B-Cdc2 kinase activity. Accumulated p21 inhibits cyclin B-Cdc2 kinase activity by direct interaction with it. Reduction of cyclin B-Cdc2 kinase activity by high level of p21 due to mutant MayD brings about making MyoD stable and the stable MyoD keeps on p21 expression and accumulated p21 also makes cyclin B-Cdc2 kinase activity weak. From such as consequent mechanism, it will keep on until wholly degradation of MyoD or p21 at G2 stage. Such a repeat cycle pathway for a long time at G2 phase delays an entry into M phase from G2 phase in cell expressing mutant MyoD. Unlike mutant MyoD, wt-MyoD makes cell enter normally into M phase from G2 phase by low expression level of p21 (Fig. 6). In this paper, this result informs direct answer about question, why does mutant MyoD lacking Cdc2 phosphorylation site delay M-phase entry and a function of p21 is a key factor for transition from G2 phase to M phase in the cell cycle. Mutant MyoD has no effect on a delay of transition from G2 to M stage in the p21 K.O mutant cell (Fig. 7). Such as result conforms that p21 do crucial role in cell cycle change from G2 to M. How can a phosphorylated MyoD downregulate p21? Even though this paper shows that wt-MyoD binds to HDAC1 stronger than mutant MyoD, this result cannot give a clear evidence enough about this question. However, phosphorylated MayD at G2 early phase is unstable and is degraded specifically in G2 late phase but not M phase. So, MyoD’s stability also functions to enter into M phase from G2 phase (Fig. 8). In conclusion, MyoD’s phosphorylation on Ser 5 and Ser 200 by cyclin B-Cdc2 destabilizes MyoD interaction with P/CAF and makes itself unstable and degradable. Such results give rise to downregulation of p21 transcription. Downregulation of p21 by a phosphorylated MyoD is important that cell enters normally from G2 phase to M phase in the cell cycle.