Farokh News and Views
advertisement

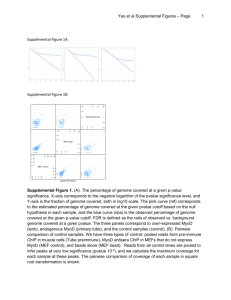
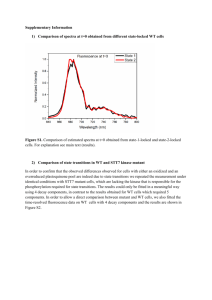
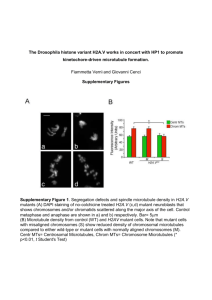
Mutant MyoD Lacking Cdc2 Phosphorylation Sites Delays M-Phase Entry -Leibovitch et al Myo D exhibits a cell cycle specific expression in myoblasts and is known to regulate genes expressed at different stages of myogenesis. It is known that Myo D phosphorylation by cyclin E-Cdk2 kinase, and it subsequent degradation is required for G1→G0 transition. There were changes observed in the Myo D levels in the G2/M phase as well suggesting a role of Myo D in G2→M transition as well. The experiment in the first figure shows the expression levels of Myo D in myoblasts in various stages of the cell cycle. There is a mobility shift in the Myo D band during the mitosis stage which returns to normal on phosphatase treatment which indicates that Myo D is phosphorylated in mitosis. There is also an overall decrease in the amount of Myo D in the M stage cell lysates indicating its degradation. The second experiment is an invitro kinase assay done using cyclin B-cdc2 kinase on point mutant forms of Myo D at S5→A5, S200→A200 and the double point mutant. The A5/A200 mutant was not phosphorylated. Next transactivation of transcription machinery by Myo D and its repression by cyclin B-cdc2 kinase was studied by expressing a Gal4 – MyoD fusion protein to drive the expression of luciferase in fibroblasts. The effect of varing levels of cycB-cdc2 kinase on luciferase activity proves that MyoD regulates transcription machinery and its activity is in turn regulated by phosphorylation at S5 and S200 sites. Transcription activator P/CAF co-immunoprecipates with MyoD in G2 stage but not in M stage and the MyoD A5/A200 mutant associates better with P/CAF than the WT. However HDAC1 association with MyoD A5/A200 mutant (which cannot be phosphorylated) is much less than WT. This indicates that phosphorylation of MyoD is essential for HDAC1 binding. P21 is known to be a downstream target of MyoD in the myogenic cells. Therefore the activation effect of MyoD on the p21 promoter was assayed using p21 promoter driven luciferase reporter gene. MyoD A5/A200 has higher activity. Cyclin B and cdc2 co-IP with p21 and the cyclin B activity (as judged by histone H1 phosphorylation) has been shown to decrease with increase in p21 levels. Effects of MyoD overexpression and expression of the A5/A200 mutant on mitosis are studied using a Tet. driven promoter and IRES – GFP reporter gene. MyoD A5/A200 mutants have a longer delay in mitotic entry as proved by FACS analysis. However this effect is not seen in a p21 mutant, which further strengthens the hypothesis that MyoD regulates the cell cycle through p21. Finally MyoD and A5/A200 mutant protein expression is quantitated in the mitotic (shake-off) fraction and the G2 (adherent) fraction. The model proposed suggests that MyoD in association with P/CAF (acetyltransferase) and causes up regulation of p21 transcription. It would have helped if they had shown MyoD actually being acetylated or observed the kinetics of p21 transcription in a P/CAF mutant. P21 then binds to cyclin B- cdc2 kinase and inactivates it in the G2 phase. This has been sufficiently proved in my opinion. In mitosis there is accumulation of cyclin B-cdc 2 kinase which phosphorylates MyoD and causes its subsequent degradation. This too is sufficiently proved. The phosphorylation of MyoD then decreases its affinity for P/CAF (needs to be sufficiently proved) and instead binds HDAC1 that inhibits p21 expression. Inhibition of p21 expression by HDAC1 needs to be proved. It would also be interesting to see the effects of constitutively phosphorylated MyoD expression in myoblasts.