Name
advertisement

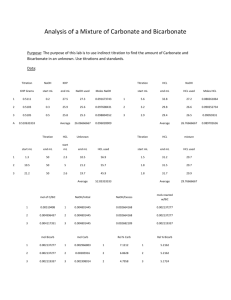
Name Chapter 12 Date Stoichiometry Class SMALL-SCALE EXPERIMENT TITRATION:DETERMINING HOW MUCH ACID IS IN A SOLUTION Small-Scale Experiment for text Section 12.2 For your course planning, you may not want to use this lab until Chapter 19. OBJECTIVES Demonstrate an understanding of the meaning of each of the following terms: qualitative, quantitative, calibration, titration, equivalence point, end point. Calibrate pipets by measuring volumes of drops. Measure the molar concentration of acid solutions by using the technique of titration. INTRODUCTION If you read the label on a typical chemical consumer product, you can often find both qualitative and quantitative information. Qualitative information answers the question, “What?” Quantitative information answers the question, “How much?” For example, a typical bottle of toilet-bowl cleaner tells you that it contains 9.5% (how much) hydrochloric acid, HCl (what). Hydrochloric acid is the “active ingredient,” the ingredient that performs the specific function for which the product is designed. Hydrochloric acid helps dissolve mineral deposits that build up on the inside of the toilet bowl. A titrations a way to measure the number of moles of a substance dissolved in a liter of solution. The resulting quantity is called the molarity of the substance and is expressed in units of moles per liter (mol/L), or simply M. An acid–base titration typically uses a known amount of base to measure an unknown amount of acid in asolution. In a typical titration, you slowly add a base with a known concentration, such as NaOH, to a measured volume of unknown acid solution, such as HCl. The neutralization reaction occurs: acid + base → salt + water HCl + NaOH → NaCl + HOH When all the acid is just neutralized by the base, the equivalence point has been reached. (The number of moles of base is equivalent to the number of moles of acid at the equivalence point.) You use the volume of base needed to reach the equivalence point to calculate how many moles of acid were neutralized by that volume of base. Because the reactants and products are colorless, it is often necessary to use an indicator to determine the equivalence point of the reaction. Phenolphthalein is an indicator that is colorless in acid solution but bright pink in basic solution. If phenolphthalein is present in the titration mixture, it will remain colorless until one drop of base neutralizes the last of the acid and makes the solution very slightly basic. At this point, called the end point, a dramatic color change from colorless to pink occurs. Notice that the end point and the equivalence point are not necessarily the same. In the case of phenolphthalein, the end point occurs when the solution turns just slightly basic, not when the solution is exactly neutral, the equivalence point. Accurate titrations require the use of an indicator that will give an end point that is very close to the equivalence point. Experiment 18 Titration: Determining How Much Acid Is in a Solution 127 Name Date Class PURPOSE In this experiment, you will use the quantitative technique of titration to determine the concentrations in moles per liter, M, of various acid samples using exactly 0.50Msodium hydroxide, NaOH. In a typical experiment, you will deliver a specific number of drops of an acid to a reaction vessel and add an indicator such as phenolphthalein. You will then count the number of drops of NaOH that is needed to turn the indicator pink. To find the volume of a drop, you will calibrate pipets by counting the number of drops each pipet delivers to fill the same size volume. You will carry out several experiments to determine what is the best way to calibrate (measure the drop size of) a pipette so that it always delivers the same size drops. Calibration is a way to correct for the different-sized drops delivered by different pipets. SAFETY Wear safety goggles, an apron, and gloves when working with corrosive chemicals. Use full small-scale pipets only for the controlled delivery of liquids. Don’t chew gum, drink, or eat in the laboratory. Never taste a chemical in the laboratory. Avoid inhaling substances that can irritate your respiratory system. MATERIALS Small-scale pipets of the following solutions: hydrochloric acid (HCl) sodium hydroxide (NaOH) phenolphthalein (phen) nitric acid (HNO3) ethanoic acid (CH3COOH) sulfuric acid (H2SO4) EQUIPMENT well plate plastic cup cotton swab EXPERIMENTAL PROCEDURE Part A. Calibrating a Small-Scale Pipet: Measuring the Relative Sizes of Drops 1. Perform a series of experiments that will answer the following questions. Work individually, as it is important that you become skilled in calibrations. Record your results in Table 18.1. a. How many drops of water from a small-scale pipet are needed to fill a well? Can you repeat this result? b. What is the best way to tell exactly when the well is full? c. Is it important to expel the air bubble before you begin? 128 Small-Scale Chemistry Laboratory Manual Name Date Class d. Try delivering drops with the pipet vertical, horizontal, and at a 45angle. At which angle do you obtain the most number of drops? The least? Which angle delivers the smallest drops? e. What will happen if you do not always hold the pipet at the same angle? f. Do different pipets give different results at the same angle? Why is it important to calibrate each different pipet you use? 2. Calibrate an HCl pipet and a NaOH pipet. Count the number of drops of each needed to fill a well for each substance. Repeat until your results are reproducible. Record your results in Table 18.2. Part B. The Titration 3. Carry out a titration of HCl as follows: a. Holding the pipet vertically, count 20 drops of HCl into a plastic cup. Take care not to touch the ends of the pipet to the cup or to the solution in the cup. Let Da equal this number of drops. b. Add one drop of phenolphthalein to the HCl in the cup. c. Add NaOH slowly, counting the number of drops of NaOH needed to obtain a stable pink end point. As you titrate, swirl the cup gently. Let Db equal this number of drops. d. Repeat Steps a–c in a clean, dry cup until your results are consistent. 4. Titrate HNO3and CH3COOH, each with NaOH in the same way. Here are some tips for accuracy and to avoid contamination. Use a clean cup. Take care not to let the ends of the pipets touch the cup or the solution in the cup. Hold the pipets vertically. As you titrate, swirl gently and count the number of drops of NaOH it takes to get a stable pink color. Clean and dry the cup before each titration. When the pipets are empty, take care to refill them with the proper solutions. Read the labels twice! Experiment 18 Titration: Determining How Much Acid Is in a Solution 129 Name Date Class EXPERIMENTAL DATA Record the results of your calibrations and titrations in Tables 18.1, 18.2, and 18.3 or in copies of the tables in your notebook. Table 18.1 Calibration of Small-Scale Pipet Trial 1 Drops to fill Trial 2 Trial 3 Vertical Horizontal 45 angle Summarize a method that will always accurately calibrate or determine the number of drops delivered by any pipet to fill a well. Table 18.2 Calibration of HCl and NaOH Pipets Trial 1 Drops to fill Trial 2 HCl NaOH 130 Small-Scale Chemistry Laboratory Manual Trial 3 Name Date Table 18.3 Titrations of Acids Drops to fill Ca Acid Class Drops NAOH to fill Cb Drops NaOH to pink Db M of acid Ma HCl HCl HNO3 HNO3 CH3COOH CH3COOH Calculate the molar concentration, Ma, of HCl and of the other two acids by substituting the following pieces of data into the expression below. Mb = molar concentration of NaOH (Mb = 0.50M in this experiment) Da = drops of acid used (20 drops in this experiment) Db = drops of NaOH to pink end point Ca = drops of acid needed to fill a well Cb = drops of NaOH needed to fill a well Ma M b Db Ca Da Cb CLEANING UP Avoid contamination by cleaning up in a way that protects you and your environment. Carefully clean the plastic cup and well plate by disposing of the liquid contents in the sink and rinsing them thoroughly with water. Dry both pieces of equipment with a paper towel. Wash your hands thoroughly with soap and water. Experiment 18 Titration: Determining How Much Acid Is in a Solution 131 Name Date Class QUESTIONS FOR ANALYSES Use what you learned in this experiment to answer the following questions. 1. What is an acid–base titration? Is titration a qualitative or quantitative method? Explain. 2. What does it mean to calibrate a pipet? Is calibration a qualitative or quantitative method? Explain. 3. Why is it important to calibrate each pipet when measuring the volumes of solutions? 4. Why is it important to repeat a calibration or a titration until the results are consistent? 5. Summarize the steps it takes to calibrate a small-scale pipet. 6. Why do your calculations of acid concentrations include the number of drops of NaOH and HCl needed to fill a well? 7. Write the chemical equation for each titration reaction you carried out in this experiment. 8. Write the balanced equation for the reaction of sulfuric acid, H 2SO4, with sodium hydroxide, NaOH. How is it different from the equations you wrote for the reactions of HCl, HNO3, and CH3COOH with NaOH? 132 Small-Scale Chemistry Laboratory Manual Name Date Class 9. Sulfuric acid, H2SO4, is an example of a diprotic acid. Explain what this means. Give an example of a triprotic acid. Give two examples of monoprotic acids. NOW IT’S YOUR TURN! 1. Let’s explore titration a little further. To carry out a “serial titration,” add four drops of HCl + one drop phen to a 1 × 12 well plate as shown below. Then add: 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 drops of NaOH. (Add the number of drops of NaOH equal to the well number.) Record your results. a. Which wells have more acid than base? Which have more base than acid? b. The end point is the point at which the indicator changes colors. Which well represents the end point of the titration? c. The equivalence point is the point at which enough base is added to just neutralize the acid. (The moles of base are “equal” to the moles of acid.) Which well represents the equivalence point? d. How does the equivalence point differ from the end point? By how many drops, at most, is the equivalence point different from the end point? 2. Design titration experiments to determine the molar concentration of H 2SO4. Be sure to calibrate each pipet you use and run each experiment at least twice so you can be confident of your answers. If you calculate the molar concentration of H2SO4 in the same way as the other acids, your result will be off by a factor of 2.Where does the factor of 2 go in the molarity calculation? 3. Some toilet-bowl cleaners are water solutions containing hydrochloric acid, HCl. Vinegar is a water solution of ethanoic acid, CH3COOH. Design experiments to determine the molar concentrations of HCl in various household products and of ethanoic acid, CH3COOH, in vinegar. Be sure to calibrate the pipets you use. Organize your results into a table like Table 18.3 and report your calculations in your laboratory notebook. (Note: Because some of the solutions are highly colored, the end point might not be pink. Try using three drops phen and look for the end point to be a distinct color change.) Experiment 18 Titration: Determining How Much Acid Is in a Solution 133